
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | SEARCH RESULT |
| ||||||||||||||||||||

|
|
|||||||||||||||||||||||||||||
RESEARCH COMMUNICATION |
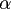 -Lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate
-Lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate
Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94720, USA; and
a Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
| ABSTRACT |
|---|
|
|
|---|
 -Lipoic
acid-supplemented old rats have improved mitochondrial function,
decreased oxidative damage, and increased metabolic rate.
-Lipoic
acid-supplemented old rats have improved mitochondrial function,
decreased oxidative damage, and increased metabolic rate.
Key Words: aging • ambulatory activity • MDA • antioxidants • liver
| INTRODUCTION |
|---|
|
|
|---|
Lipoic acid is a disulfide compound found naturally in mitochondria as
the coenzyme for pyruvate dehydrogenase and  -ketoglutarate
dehydrogenase. It has been used as therapy for many diseases associated
with impaired energy utilization, such as type II diabetes
(7)
and diabetic polyneuropathies 8, 9)
.
Dietary supplementation also increases unbound lipoic acid, which can
act as a potent antioxidant and ameliorate oxidative stress both
in vitro and in vivo 10-15)
. To a
degree, aging results in the same type(s) of metabolic impairment and
increased oxidative stress as shown in these conditions.
-ketoglutarate
dehydrogenase. It has been used as therapy for many diseases associated
with impaired energy utilization, such as type II diabetes
(7)
and diabetic polyneuropathies 8, 9)
.
Dietary supplementation also increases unbound lipoic acid, which can
act as a potent antioxidant and ameliorate oxidative stress both
in vitro and in vivo 10-15)
. To a
degree, aging results in the same type(s) of metabolic impairment and
increased oxidative stress as shown in these conditions.
Though its ability to improve energy metabolism (16) and lower oxidative stress 11-15) for certain disease states has been described, it is not known whether lipoic acid supplementation may also reverse energy-linked metabolic deficits or reduce the increased oxidative stress seen in aging. The purpose of this study was twofold: to 1) determine whether (R)-lipoic acid supplementation increased cellular and general metabolic activity in old rats, and 2) examine whether this supplementation affected hepatocellular antioxidant status, oxidant production, and oxidative damage.
We show that supplementing old rats with 0.5% (w/w) lipoic acid for 2 wk partially reverses the age-associated loss of mitochondrial function, an increase in oxidative stress, and the damage and decline in general metabolic activity.
| MATERIALS AND METHODS |
|---|
|
|
|---|
Animals
Rats (Fisher 344, virgin male, outbred albino), both young (3–5
months; Simonsen, Gilroy, Calif.) and old (24–26 months; National
Institute of Aging animal colonies), were acclimatized in the Berkeley
animal facilities for at least 1 wk prior to experimentation. The
AIN-93M standard diet or one supplemented with 0.5% (w/w)
(R)-lipoic acid, and water ad libitum was given throughout.
Cell isolation
Liver tissue was dispersed into single cells by collagenase
perfusion (17)
. Cell number was assessed using a
hemocytometer, and viability (typically greater than 90% in both age
groups) was determined by Trypan blue exclusion.
Mitochondrial membrane potential
The average mitochondrial membrane potential in intact
hepatocytes was measured by flow cytometry using R123 as the
fluorescent probe (4)
. Hepatocytes (2.0x106
cells) were incubated with R123 (0.01 mg/ml) for 30 min at 37°C, then
subjected to flow cytometry using an instrument constructed according
to the design of Steinkamp et al. (18)
. Nonspecific light
scatter was subtracted and cells showing a particular fluorescence were
quantified.
Oxygen consumption studies
Hepatocellular oxygen consumption was analyzed using a YSI 5300
oxygen electrode and monitor (Yellow Springs, Ohio).
DCFH measurement
Formation of oxidants in cells were determined by assaying the
fluorescence of 2',7'-dichlorofluorescein, the oxidation product of
DCFH (19)
. Quadruplicate samples were routinely analyzed.
Fluorescence was monitored using a Cytofluor 2350 fluorescent
measurement system (Millipore, Bedford, Mass.) using standard
fluorescein filters and Cytocalc software. Oxygen consumption was
measured and data were expressed as the fluorescence per µM
O2 consumed/106 cells.
GSH analysis
Reduced GSH was measured by high-performance liquid
chromatography (HPLC) as described by Reed et al. (20)
.
Briefly, cells were mixed with 5-sulfosalicylic acid [7.5% (w/v),
final concentration] and the samples were spun for 1 min at 13,000 RPM
in a microcentrifuge to remove denatured debris. An aliquot of the
supernatant was added to 100 µl of 1M Trizma Base buffer (pH 8),
followed by addition of 100 µl of 40 mM fresh aqueous iodoacetic acid
(4 µmol). The reaction mixture was brought to pH 8 with
NaHCO3 and dinitrophenyl derivatives were made by addition
of 500 µl of 2,4-dinitrofluorobenzene [1.5% (v/v) in absolute
ethanol] and 100 to 200 µl of K2CO3. The
resultant derivatives were separated on a 10 µm Ultrasphere-amine
column (4.6 mmx25 cm) using a Waters HPLC system and solvents, as
described (20)
. GSH was quantified relative to standards.
Ascorbic acid analysis
Total ascorbic acid quantification was performed after reduction
with dithiothreitol, as described (21)
. The samples were
placed in a chilled (2°C) auto sampler for analysis. The system used
for separation was reversed-phase HPLC (Hewlett-Packard, Mountain View,
Calif.) with coulometric detection (ESA Inc., Bedford, Mass.). The peak
area corresponding to ascorbic acid was integrated using HP ChemStation
software (Hewlett-Packard).
Malondialdehyde analysis
Lipid peroxidation was assayed using a recently developed
sensitive and specific gas chromatography-mass spectrometry method for
malondialdehyde (MDA) 22, 23)
. Briefly, the hepatocytes
were lysed with phosphate-buffered saline containing 2.8 mM butylated
hydroxytoluene and 1% sodium dodecyl sulfate, pH 7.4. The
protein-bound MDA was hydrolyzed with H2SO4.
MDA was converted into a stable derivative, using pentafluorophenyl
hydrazine at room temperature, and the derivative was detected with a
Hewlett Packard 5890 Series II gas chromatograph interfaced to a 5989
mass spectrometry system equipped with a J & W Scientific DBWAX
capillary column (15 mx0.25 mm i. d., 0.25 µm film thickness) in the
negative chemical ionization mode. The results were indexed with
protein, which was measured with a modified Lowry method.
Ambulatory activity tests
Each night rats were moved from group housing to individual
cages (48 cm lx25 wx20 h) at least 4 h prior to the
quantification of ambulatory parameters. The room was on a 12 h
light/dark cycle (lights on 6 AM to 6 PM). At 8
PM, a very low light illuminated the test subjects for
video tracking. Quantification began at 9 PM and continued
for 4 h. One hour later the low light was turned off and the room
remained in total darkness until 6 AM, when the standard
light/dark cycle began. A video signal from a camera suspended directly
above the individual cages was fed directly into a Videomex-V (Columbus
Instruments, Columbus, Ohio) computer system running the Multiple
Objects Multiple Zones software. The system quantified ambulatory
activity parameters and was calibrated to report distance traveled in
centimeters. In addition to total distance traveled, the time each
subject spent in ambulatory (locomotor), stereotypic (grooming), and
resting (nonmovement) activity was recorded by an IBM computer. No
additional modifications (such as fur dying) were needed to
continuously track the subjects. At 9 AM animals were
removed from individual housing and returned to group housing. Results
are shown as the mean centimeters traveled per hour ±SEM.
The ambulatory activity of each rat was recorded before lipoic acid supplementation and for two consecutive nights. After lipoic acid supplementation and for two consecutive nights, the same spontaneous locomotor parameters were determined. With this design, each rat acted as its own control. After measurement of ambulatory activity, some lipoic acid-supplemented animals were placed on an AIN-93M diet for three additional weeks and activity was again measured.
Statistical analysis
Statistical significance was determined using the paired
Student's t test or one way analysis of variance. Results
are expressed as the mean ±SEM. A P value of
less than 0.05 was considered significant.
| RESULTS |
|---|
|
|
|---|
|
The mitochondrial membrane potential in hepatocytes was measured using R123 fluorescence in order to test whether the lipoic acid-induced increase in O2 consumption in hepatocytes from old rats was attributable to enhanced mitochondrial function. The average mitochondrial membrane potential in the majority of hepatocytes from old rats has previously been shown to be approximately 40% that of hepatocytes from young rats, a significant loss (P<0.02; N=8) of the driving force for ATP production (4) . (R)-Lipoic acid supplementation caused the mitochondrial membrane potential to increase by 50.0% ±7.9 (N=4) over that of unsupplemented old rats, a marked improvement (P<0.03), but still significantly lower (P<0.04) when compared to cells from young untreated rats. Thus, (R)-lipoic acid supplementation partially improves mitochondrial function in old rats and may alleviate some loss of metabolic activity associated with aging.
To determine whether (R)-lipoic acid improved metabolic activity on a physiological basis, we quantified ambulatory activity in rats with and without lipoic acid treatment. Ambulatory activity declined almost threefold with age (Fig. 1 ). This significant decline was partially reversed by (R)-lipoic acid supplementation, which increased ambulatory activity by twofold over untreated old animals (P<0.0005). Activity in treated old rats compared to untreated young rats was lower, but not significantly so (P<0.06). Feeding (R)-lipoic acid to young rats also increased ambulatory activity, but this increase was not significant.
|
To confirm the effect of (R)-lipoic acid on metabolic activity, a three-staged feeding regimen using the same aged rats was designed. Ambulatory activity was monitored 1) after feeding an AIN-93M diet for 2 wk, 2) after feeding (R)-lipoic acid supplemented AIN-93M diet for 2 wk, and 3) after feeding an AIN-93M diet (without lipoic acid) for three additional weeks. The results of this experiment showed that during the lipoic acid supplementation period, ambulatory activity again was significantly higher (P<0.03; Fig. 2 ). Removal of (R)-lipoic acid from the diet reversed this improvement (Fig. 2) . Control experiments where the AIN-93M was given to old rats throughout the study, but otherwise treated similarly to the experimental group, showed no change in ambulatory activity (data not shown). Thus, (R)-lipoic acid significantly increases overall physiological activity among old rats.
|
Effect of lipoic acid supplementation on oxidant stress
(R)-Lipoic acid acts as a cofactor in several
mitochondrial enzyme complexes, but is also a powerful antioxidant and
increases levels of other endogenous antioxidants when given as a
supplement. The effect of (R)-lipoic acid supplementation on
antioxidant status, oxidant production, and levels of oxidative damage
in hepatocytes from old rats was examined.
Hepatocellular GSH and ascorbic acid concentrations were measured to determine whether these low molecular weight antioxidants declined with age. Both GSH and ascorbic acid levels were significantly lower (P<0.05) in hepatocytes from old compared to young rats, with declines of 23% and 50%, respectively (Fig. 3A, B ). Supplementation of (R)-lipoic acid for 2 wk prior to cell isolation restored the level of antioxidants to that of young animals. In both young and old rats, hepatocellular GSH levels were significantly higher vs. their corresponding controls (P<0.03; Fig. 3A ); GSH levels were more than twofold higher in old rats than in unsupplemented animals. Lipoic acid supplementation also restored the cellular ascorbic acid levels to that of young rats (Fig. 3B ). Thus, (R)-lipoic acid reverses the age-associated decline in endogenous low molecular weight antioxidants, and therefore may lower the increased risk for oxidative damage that occurs during aging.
|
We previously showed that hepatocytes from old rats have a higher rate of oxidant production per oxygen consumed, as measured by the fluorescence formed on oxidizing DCFH (4) . To determine whether dietary (R)-lipoic acid could lower the increased rate of oxidant production seen in aging, we measured the fluorescence in supplemented animals and their corresponding controls. Cells from old rats had significantly higher oxidant production (P<0.005), nearly twofold more than that in young rats (Fig. 4 ). In contrast, oxidant production was lowered in cells from lipoic acid-treated old rats to a level not significantly different from those of untreated young rats (Fig. 4) .
|
To gauge whether the lipoic acid-induced decline in oxidant production translated into lower levels of oxidative damage, MDA was measured as an indicator of cellular lipid peroxidation 22, 23) . Hepatocytes from unsupplemented old rats had fivefold more MDA than the cells from young rats (P<0.01; Fig. 5 ). (R)-Lipoic acid supplementation reduced MDA levels markedly in old rats (P<0.01) (Fig. 5) . Although this decline in lipid peroxidation was substantial, the levels observed in the lipoic acid-supplemented old rats were still significantly higher than those found in cells from unsupplemented young rats (P=0.05). MDA levels were higher in lipoic acid-supplemented young animals, but this increase was not significant. Thus, (R)-lipoic acid supplementation markedly lowers oxidant production and the attendant increase in oxidative damage associated with aging.
|
| DISCUSSION |
|---|
|
|
|---|
 -keto acid
dehydrogenases and specifically reduced to dihydrolipoic acid, a
powerful antioxidant, via mitochondrial lipoamide dehydrogenase. There
is evidence that (R)-lipoic acid supplementation may be more
potent than either the racemic mixture (the form sold commercially as
-keto acid
dehydrogenases and specifically reduced to dihydrolipoic acid, a
powerful antioxidant, via mitochondrial lipoamide dehydrogenase. There
is evidence that (R)-lipoic acid supplementation may be more
potent than either the racemic mixture (the form sold commercially as
 -lipoic acid) or (S)-enantiomer, and thus a more relevant
supplement for this study. Addition of (R)-lipoic acid
increases ATP synthesis and aortic blood flow during reoxygenation
after hypoxia in a working heart model (25)
. The
(S)-enantiomer had no effect on ATP synthesis and improved
blood flow at only 10-fold the effective dose of (R)-lipoic
acid. Packer and colleagues (26)
also showed that
(R)-lipoic acid significantly reduced
buthionine-S,R-sulfoximine-induced cataract formation, but
(S)-lipoic acid had little effect at the same concentration.
(R)-Lipoic acid increased glucose uptake and the number of
glucose transporters in muscle tissue much more effectively than
(S)-lipoic acid (27)
. The
(R)-enantiomer more effectively chelated copper and
prevented copper-induced lipid peroxidation (28)
.
-lipoic acid) or (S)-enantiomer, and thus a more relevant
supplement for this study. Addition of (R)-lipoic acid
increases ATP synthesis and aortic blood flow during reoxygenation
after hypoxia in a working heart model (25)
. The
(S)-enantiomer had no effect on ATP synthesis and improved
blood flow at only 10-fold the effective dose of (R)-lipoic
acid. Packer and colleagues (26)
also showed that
(R)-lipoic acid significantly reduced
buthionine-S,R-sulfoximine-induced cataract formation, but
(S)-lipoic acid had little effect at the same concentration.
(R)-Lipoic acid increased glucose uptake and the number of
glucose transporters in muscle tissue much more effectively than
(S)-lipoic acid (27)
. The
(R)-enantiomer more effectively chelated copper and
prevented copper-induced lipid peroxidation (28)
. We did not measure hepatic tissue concentrations of (R)-lipoic acid or dihydrolipoic acid after oral supplementation. However, the characteristics of its uptake and tissue distribution in the rat have previously been examined, although not on an age-related basis. Lipoic acid is rapidly absorbed in the gastrointestinal tract but is subject to considerable presystemic elimination 29, 30) . Between 27 to 34% of orally administered lipoic acid is available for tissue uptake, and the liver is one of the major organs of clearance (31) . Studies where radiolabeled lipoic acid was infused into rats revealed that the liver has a high capacity for both uptake and accumulation of lipoic acid (32) . Thus, dietary supplementation of lipoic acid would be expected to elevate hepatocellular lipoic acid concentrations considerably in both young and old rats, though its release from the liver may also be rapid.
A pharmacological dose of (R)-lipoic acid was given to maximize the possibility of observing whether it could affect metabolic activity and lower the increased oxidative stress evident in old rats. Even though the supplemental dose given was relatively high, it was considerably lower than the reported LD50 concentration for (R)- or (R,S)-lipoic acid for old rats (24) . Rats fed the lipoic acid-supplemented diet for 2 wk exhibited no adverse side effects other than a small amount of weight loss, which we attribute to increased general metabolic activity. We are currently determining whether lower levels of (R)-lipoic acid in the diet would be equally effective in partially restoring metabolic function and decreasing oxidative stress in old rats.
We demonstrate that lipoic acid supplementation of old rats markedly improves the average mitochondrial membrane potential and restores the cellular oxygen consumption (Table 1) in hepatocytes to that of young rats. Rats on this feeding regimen were significantly more active, which further shows that (R)-lipoic acid acts physiologically to increase general metabolic activity. While the underlying causes for this increased energy metabolism were not explored, it is plausible that lipoic acid improves mitochondrial function through a number of mechanisms. Administration of lipoic acid stimulates insulin-dependent and independent glucose uptake into cells (33) and also enhances nonoxidative and oxidative glucose metabolism. Reduced (R)-lipoic acid has also been shown to increase ATP synthase activity (16) , which in combination with increased glucose utilization would be expected to enhance overall cellular metabolism. Finally, as a potent antioxidant, dihydrolipoic acid may also maintain critical thiol groups in a reduced state and allow mitochondrial protein carriers to function more effectively (16) .
We also show that feeding (R)-lipoic acid significantly attenuates the age-related increase in hepatocellular oxidant production as well as lipid peroxidation. This reduction in oxidative stress may be directly attributable to increased unbound dihydrolipoic acid or indirectly due to higher levels of other antioxidants. Lipoic acid raises GSH values by increasing cysteine availability (12) , which is the rate-limiting factor in its biosynthesis. Lipoic acid decreases levels of GSH protein-mixed disulfides (34) . Lipoic acid also causes faster ascorbic acid recycling (13) . This may be important because ascorbic acid recycling in times of oxidative insult is markedly impaired in cells from old rats (14) and (R)-lipoic acid supplementation reverses this decline (14) . Thus, feeding lipoic acid generally improves cellular antioxidant status, which declines with age.
 -Lipoic acid has been used as a therapeutic agent in humans,
especially for diabetes 7, 9, 35)
as well as certain
toxicological and pathological conditions of the liver 24, 36, 37)
. However, little is known about whether
(R)-lipoic acid may be an effective anti-aging supplement or
therapy for certain diseases in humans. Our present findings using rats
would suggest that (R)-lipoic acid supplementation may be a
safe and effective means to improve general metabolic activity and
increase antioxidant status, affording increased protection against
external oxidative and xenobiotic insults with age.
-Lipoic acid has been used as a therapeutic agent in humans,
especially for diabetes 7, 9, 35)
as well as certain
toxicological and pathological conditions of the liver 24, 36, 37)
. However, little is known about whether
(R)-lipoic acid may be an effective anti-aging supplement or
therapy for certain diseases in humans. Our present findings using rats
would suggest that (R)-lipoic acid supplementation may be a
safe and effective means to improve general metabolic activity and
increase antioxidant status, affording increased protection against
external oxidative and xenobiotic insults with age.
Other critical metabolites that become limiting due to age-associated metabolic changes may also be beneficial as dietary supplements. A number of studies report that administration of acetyl-L-carnitine (ALCAR), a derivative of carnitine involved in fatty acid transport into mitochondria, enhanced mitochondrial function in aged tissue 38-42) . We previously found (43) that ALCAR fed to old rats restores decayed mitochondria for cardiolipin content, membrane potential, and oxygen consumption and restores ambulatory activity of the rats. However, ALCAR supplementation also increased the rate of oxidant production, oxidative damage, and decreased cellular antioxidant levels (43) . This indicated that ALCAR supplementation improved mitochondrial electron flux but did not reverse the increased inefficiency of electron transport. In a separate study (T. M. Hagen et al., unpublished results), we find that feeding ALCAR in combination with lipoic acid to old rats effectively increases mitochondrial metabolism without an increase in oxidative stress. Long-term feeding studies are warranted to determine whether these observed changes in mitochondria will significantly diminish decline in energy metabolism and the increased oxidative stress evident in aging.
| ACKNOWLEDGMENTS |
|---|
| FOOTNOTES |
|---|
1 Present address: Linus Pauling Institute,
Oregon State University, 571 Weniger Hall, Corvallis, OR 97331, USA. ![]()
2 Department of Pharmacology and Pathobiology, Royal
Veterinary and Agricultural University, Copenhagen, Denmark. ![]()
4 Abbreviations: ALCAR, acetyl-L-carnitine; GSH: glutathione; HPLC, high-performance liquid chromatography; DCFH: 2',7'-dichlorofluorescin diacetate; MDA: malondialdehyde; R123, rhodamine 123.
Received for publication August 3, 1998.
Revision received October 15, 1998.
| REFERENCES |
|---|
|
|
|---|
 -lipoic acid: A 3-week multicentre randomized controlled trial (ALADIN study). Diabetologia 1995;38:1425-1433.[Medline]
-lipoic acid: A 3-week multicentre randomized controlled trial (ALADIN study). Diabetologia 1995;38:1425-1433.[Medline]
 -lipoic acid supplementation. FASEB J 1998;12:1183-1189.
-lipoic acid supplementation. FASEB J 1998;12:1183-1189. -Lipoic acid in liver metabolism and disease. Free Rad. Biol. Med. 1998;24:1023-1039.[Medline]
-Lipoic acid in liver metabolism and disease. Free Rad. Biol. Med. 1998;24:1023-1039.[Medline]
 -lipoic acid from in situ ligated segments of the gastrointestinal tract of the rat. Arzneim. Forsch. Drug Res. 1995;45:293-299.
-lipoic acid from in situ ligated segments of the gastrointestinal tract of the rat. Arzneim. Forsch. Drug Res. 1995;45:293-299.
This article has been cited by other articles:
 |
J. A. Lemon, D. R. Boreham, and C. D. Rollo A Complex Dietary Supplement Extends Longevity of Mice J. Gerontol. A Biol. Sci. Med. Sci., March 1, 2005; 60(3): 275 - 279. [Abstract] [Full Text] [PDF] |
||||
 |
J. LIU, E. HEAD, H. KURATSUNE, C. W. COTMAN, and B. N. AMES Comparison of the Effects of l-Carnitine and Acetyl-l-Carnitine on Carnitine Levels, Ambulatory Activity, and Oxidative Stress Biomarkers in the Brain of Old Rats Ann. N.Y. Acad. Sci., November 1, 2004; 1033(1): 117 - 131. [Abstract] [Full Text] [PDF] |
||||
 |
B. N. AMES and J. LIU Delaying the Mitochondrial Decay of Aging with Acetylcarnitine Ann. N.Y. Acad. Sci., November 1, 2004; 1033(1): 108 - 116. [Abstract] [Full Text] [PDF] |
||||
 |
B. N. Ames Supplements and Tuning Up Metabolism J. Nutr., November 1, 2004; 134(11): 3164S - 3168S. [Full Text] [PDF] |
||||
 |
B. F. Terjesen, K. Park, M. B. Tesser, M. C. Portella, Y. Zhang, and K. Dabrowski Lipoic Acid and Ascorbic Acid Affect Plasma Free Amino Acids Selectively in the Teleost Fish Pacu (Piaractus mesopotamicus) J. Nutr., November 1, 2004; 134(11): 2930 - 2934. [Abstract] [Full Text] [PDF] |
||||
 |
J. H. Bauer, S. Goupil, G. B. Garber, and S. L. Helfand An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster PNAS, August 31, 2004; 101(35): 12980 - 12985. [Abstract] [Full Text] [PDF] |
||||
 |
B. N. AMES Delaying the Mitochondrial Decay of Aging Ann. N.Y. Acad. Sci., June 1, 2004; 1019(1): 406 - 411. [Abstract] [Full Text] [PDF] |
||||
 |
P. Arivazhagan, S. R. Panneerselvam, and C. Panneerselvam Effect of DL-{alpha}-Lipoic Acid on the Status of Lipid Peroxidation and Lipids in Aged Rats J. Gerontol. A Biol. Sci. Med. Sci., September 1, 2003; 58(9): B788 - 791. [Abstract] [Full Text] [PDF] |
||||
 |
J.A. Lemon, D.R. Boreham, and C.D. Rollo A Dietary Supplement Abolishes Age-Related Cognitive Decline in Transgenic Mice Expressing Elevated Free Radical Processes Experimental Biology and Medicine, July 1, 2003; 228(7): 800 - 810. [Abstract] [Full Text] [PDF] |
||||
 |
B. N. Ames An Enthusiasm for Metabolism J. Biol. Chem., February 14, 2003; 278(7): 4369 - 4380. [Full Text] [PDF] |
||||
 |
C. A. Williams, R. M. Hoffman, D. S. Kronfeld, T. M. Hess, K. E. Saker, and P. A. Harris Lipoic Acid as an Antioxidant in Mature Thoroughbred Geldings: A Preliminary Study J. Nutr., June 1, 2002; 132(6): 1628S - 1631. [Abstract] [Full Text] [PDF] |
||||
 |
A. D. N. J. DE GREY, B. N. AMES, J. K. ANDERSEN, A. BARTKE, J. CAMPISI, C. B. HEWARD, R. J. M. McCARTER, and G. STOCK Time to Talk SENS: Critiquing the Immutability of Human Aging Ann. N.Y. Acad. Sci., April 1, 2002; 959(1): 452 - 462. [Abstract] [Full Text] [PDF] |
||||
 |
J. LIU, H. ATAMNA, H. KURATSUNE, and B. N. AMES Delaying Brain Mitochondrial Decay and Aging with Mitochondrial Antioxidants and Metabolites Ann. N.Y. Acad. Sci., April 1, 2002; 959(1): 133 - 166. [Abstract] [Full Text] [PDF] |
||||
 |
B. N Ames, I. Elson-Schwab, and E. A Silver High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): relevance to genetic disease and polymorphisms Am. J. Clinical Nutrition, April 1, 2002; 75(4): 616 - 658. [Abstract] [Full Text] [PDF] |
||||
 |
C. Zhao and K. Hemminki The in vivo levels of DNA alkylation products in human lymphocytes are not age dependent: an assay of 7-methyl- and 7-(2-hydroxyethyl)-guanine DNA adducts Carcinogenesis, February 1, 2002; 23(2): 307 - 310. [Abstract] [Full Text] [PDF] |
||||
 |
J. Liu, E. Head, A. M. Gharib, W. Yuan, R. T. Ingersoll, T. M. Hagen, C. W. Cotman, and B. N. Ames Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid PNAS, February 19, 2002; 99(4): 2356 - 2361. [Abstract] [Full Text] [PDF] |
||||
 |
J. Liu, D. W. Killilea, and B. N. Ames Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid PNAS, February 19, 2002; 99(4): 1876 - 1881. [Abstract] [Full Text] [PDF] |
||||
 |
T. M. Hagen, J. Liu, J. Lykkesfeldt, C. M. Wehr, R. T. Ingersoll, V. Vinarsky, J. C. Bartholomew, and B. N. Ames Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress PNAS, February 19, 2002; 99(4): 1870 - 1875. [Abstract] [Full Text] [PDF] |
||||
 |
B. N. AMES Micronutrient Deficiencies: A Major Cause of DNA Damage Ann. N.Y. Acad. Sci., January 1, 1999; 889(1): 87 - 106. [Abstract] [Full Text] [PDF] |
||||
 |
D. Harman Aging: Overview Ann. N.Y. Acad. Sci., April 1, 2001; 928(1): 1 - 21. [Abstract] [Full Text] [PDF] |
||||
 |
V. Saengsirisuwan, F. R. Perez, T. R. Kinnick, and E. J. Henriksen Effects of exercise training and antioxidant R-ALA on glucose transport in insulin-sensitive rat skeletal muscle J Appl Physiol, January 1, 2002; 92(1): 50 - 58. [Abstract] [Full Text] [PDF] |
||||
 |
H. ATAMNA, C. ROBINSON, R. INGERSOLL, H. ELLIOTT, and B. N. AMES N-t-Butyl hydroxylamine is an antioxidant that reverses age-related changes in mitochondria in vivo and in vitro FASEB J, October 1, 2001; 15(12): 2196 - 2204. [Abstract] [Full Text] |
||||
 |
A. Mansouri, C. Demeilliers, S. Amsellem, D. Pessayre, and B. Fromenty Acute Ethanol Administration Oxidatively Damages and Depletes Mitochondrial DNA in Mouse Liver, Brain, Heart, and Skeletal Muscles: Protective Effects of Antioxidants J. Pharmacol. Exp. Ther., August 1, 2001; 298(2): 737 - 743. [Abstract] [Full Text] [PDF] |
||||
 |
Q. Jiang, I. Elson-Schwab, C. Courtemanche, and B. N. Ames gamma -Tocopherol and its major metabolite, in contrast to alpha -tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells PNAS, October 10, 2000; 97(21): 11494 - 11499. [Abstract] [Full Text] [PDF] |
||||
| ||||||||||||||||||||||||||||||||||||||||
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | SEARCH RESULT |