| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
| ||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
J. Biol. Chem., Vol. 277, Issue 30, 26893-26903, July 26, 2002
| ||||||||||||||||||||||||||||||||||||||||||||||||
 ,
, ,
,

From the  Department of Medical Microbiology,
University of Manitoba and National Microbiology Laboratory, Health
Canada, Winnipeg, Manitoba R3E 0W3, Canada and the
§ Laboratory of Intracellular Parasites and
¶ Laboratory of Human Bacterial Pathogenesis, Rocky Mountain
Laboratories, NIAID, National Institutes of Health,
Hamilton, Montana 59840
Department of Medical Microbiology,
University of Manitoba and National Microbiology Laboratory, Health
Canada, Winnipeg, Manitoba R3E 0W3, Canada and the
§ Laboratory of Intracellular Parasites and
¶ Laboratory of Human Bacterial Pathogenesis, Rocky Mountain
Laboratories, NIAID, National Institutes of Health,
Hamilton, Montana 59840
Received for publication, April 23, 2002, and in revised form, May 7, 2002
| |
ABSTRACT |
|---|
|
|
|---|
Here we report the cloning and
sequencing of a region of the chlamydiae chromosome termed the
"plasticity zone" from all the human serovars of C. trachomatis containing the tryptophan biosynthesis genes. Our
results show that this region contains orthologues of the tryptophan
repressor as well as the  and
and  subunits of tryptophan synthase.
Results from reverse transcription-PCR and Western blot analyses
indicate that the trpBA genes are transcribed, and protein
products are expressed. The TrpB sequences from all serovars are highly conserved. In comparison with other tryptophan synthase
subunits of tryptophan synthase.
Results from reverse transcription-PCR and Western blot analyses
indicate that the trpBA genes are transcribed, and protein
products are expressed. The TrpB sequences from all serovars are highly conserved. In comparison with other tryptophan synthase  subunits, the chlamydial TrpB subunit retains all
conserved amino acid residues required for
subunits, the chlamydial TrpB subunit retains all
conserved amino acid residues required for  reaction activity. In
contrast, the chlamydial TrpA sequences display numerous mutations,
which distinguish them from TrpA sequences of all other prokaryotes. All ocular serovars contain a deletion mutation resulting in a truncated TrpA protein, which lacks
reaction activity. In
contrast, the chlamydial TrpA sequences display numerous mutations,
which distinguish them from TrpA sequences of all other prokaryotes. All ocular serovars contain a deletion mutation resulting in a truncated TrpA protein, which lacks  reaction activity. The TrpA protein from the genital serovars retains conserved amino acids required for catalysis but has mutated several active site residues involved in substrate binding. Complementation analysis in
Escherchia coli strains, with defined mutations in
tryptophan biosynthesis, and in vitro enzyme activity data,
with cloned TrpB and TrpA proteins, indicate these mutations result in
a TrpA protein that is unable to utilize indole glycerol 3-phosphate as
substrate. In contrast, the chlamydial TrpB protein can carry out the
reaction activity. The TrpA protein from the genital serovars retains conserved amino acids required for catalysis but has mutated several active site residues involved in substrate binding. Complementation analysis in
Escherchia coli strains, with defined mutations in
tryptophan biosynthesis, and in vitro enzyme activity data,
with cloned TrpB and TrpA proteins, indicate these mutations result in
a TrpA protein that is unable to utilize indole glycerol 3-phosphate as
substrate. In contrast, the chlamydial TrpB protein can carry out the
 reaction, which catalyzes the formation of tryptophan from indole
and serine. The activity of the chlamydial Trp B protein differs from
that of the well characterized E. coli and
Salmonella TrpBs in displaying an absolute requirement for
full-length TrpA. Taken together our data indicate that genital, but
not ocular, serovars are capable of utilizing exogenous indole for
the biosynthesis of tryptophan.
reaction, which catalyzes the formation of tryptophan from indole
and serine. The activity of the chlamydial Trp B protein differs from
that of the well characterized E. coli and
Salmonella TrpBs in displaying an absolute requirement for
full-length TrpA. Taken together our data indicate that genital, but
not ocular, serovars are capable of utilizing exogenous indole for
the biosynthesis of tryptophan.
| |
INTRODUCTION |
|---|
|
|
|---|
Members of the genus Chlamydia are obligate intracellular bacteria that possess a unique biphasic developmental cycle consisting of an extracellular, infectious, but metabolically inactive elementary body (EB)1 and an intracellular, non-infectious, replicative form called the reticulate body (RB). Chlamydial infection involves the attachment of the EB to a host cell and its subsequent internalization into a membrane-bound vesicle known as the chlamydial inclusion. Within this inclusion the EB differentiates into an RB, which then multiplies by binary fission. The daughter RBs then redifferentiate into EBs that are able to initiate new rounds of infection after release by host cell lysis (1).
Chlamydia consists of three species that are important pathogens of humans. Chlamydia psittaci strains are primarily pathogens of birds and lower animals, but humans are occasional hosts of avian-acquired psittacosis (2, 3). The two major pathogens of humans are Chlamydia trachomatis and Chlamydia pneumoniae. C. pneumoniae is an important cause of community-acquired pneumoniae (4) and has been linked to the etiology of chronic heart disease (5-7). C. trachomatis comprises a family of antigenically related yet divergent organisms serologically classified into 15 distinct serovars based on antigenic variation of the major outer membrane protein of the organism (8). Curiously, the 15 different C. trachomatis serovars exhibit an extraordinary specificity in tissue tropism. For example, serovars A, B, Ba, and C are pathogens of the eye, where they infect columnar epithelial cells of the conjunctivae causing trachoma, a chronic inflammatory disease that is the leading cause of preventable blindness in the world (2, 3, 9). The trachoma serovars are rarely isolated from the genital tract. On the other hand, serovars D-K are sexually transmitted pathogens that infect columnar epithelial cells of the genital tract (2, 3). These infections are the most common bacterial cause of sexually transmitted disease and in females cause pelvic inflammatory disease. The sexually transmitted disease serovars can cause neonatal conjunctivitis but have not been associated with blinding trachoma. Furthermore, although infections with both ocular (A-C) and genital serovars (D-K) are non-invasive and are restricted to the mucosal epithelium, those caused by the sexually transmitted lymphogranuloma venereum (LGV) serovars (L1, L2, and L3) are invasive (2, 3). The LGV strains penetrate the submucosal tissue, infect monocytes and macrophages, and disseminate to the local draining lymph nodes, where they produce a chronic granulomatous disease. The factor(s) that controls the non-invasive/invasive properties of these genital serovars has been correlated with the production of a chlamydial cytotoxin (10); however, virulence factors that decide the distinctive ocular and genital tract tissue tropisms have yet to be discovered.
T cells play an important role in the development of adaptive immunity
against C. trachomatis mucosal infection, and interferon 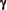 (IFN-
(IFN-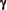 ) is key to this protective function (11-13). The mechanism by
which IFN-
) is key to this protective function (11-13). The mechanism by
which IFN-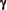 controls infection in vitro is by interfering
with the replicative capacity of the parasite (14, 15). Through binding
of the IFN-
controls infection in vitro is by interfering
with the replicative capacity of the parasite (14, 15). Through binding
of the IFN-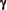 receptor, IFN-
receptor, IFN-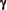 transcriptionally activates the
expression of indoleamine-2,3-dioxygenase, which degrades L-tryptophan to L-kynurenine (16, 17). This
cytokine-mediated host cell response deprives intracellular chlamydial
RBs of tryptophan, which ultimately prevents their growth and
replicative capabilities. Treatment of epithelial cells with high
levels of IFN-
transcriptionally activates the
expression of indoleamine-2,3-dioxygenase, which degrades L-tryptophan to L-kynurenine (16, 17). This
cytokine-mediated host cell response deprives intracellular chlamydial
RBs of tryptophan, which ultimately prevents their growth and
replicative capabilities. Treatment of epithelial cells with high
levels of IFN-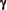 completely inhibits growth, whereas subinhibitory
concentrations induce the development of morphologically aberrant
viable RB forms that have been implicated in the development of
persistence (15).
completely inhibits growth, whereas subinhibitory
concentrations induce the development of morphologically aberrant
viable RB forms that have been implicated in the development of
persistence (15).
The complete genomic sequence of several Chlamydiaceae has been
determined, including C. trachomatis serovars D (18) and MoPn (19), C. pneumoniae strains CWL029 (20), AR39 (19), and
J138 (21). and C. psittaci strain GPIC (www.tigr.org). In addition, partial sequence information is available for C. trachomatis serovar L2 (chlamydia-www.Berkeley.edu:4231). The gene
order and content among these organisms are remarkably similar, with
the exception of a region termed the plasticity zone, which has
undergone genetic reorganization to a greater extent than the rest of
the chromosome (19). Genes encoding enzymes required for the
biosynthesis of tryptophan are found within the plasticity zone.
However, the complement of trp genes within this region
varies among the chlamydial species characterized to date. C. psittaci GPIC contains all of the genes of the tryptophan
biosynthesis pathway with the exception of the first two enzymes
encoded by trpE/G. In contrast, C. trachomatis serovar MoPn and C. pneumoniae do not
encode any trp genes in the plasticity zone. Interestingly,
C. trachomatis serovars D and L2 contain only a subset of
trp genes in their plasticity zone, trpR,
encoding a putative tryptophan repressor, and trpA and
trpB, respectively, encoding homologues of the  (TrpA)
and
(TrpA)
and  (TrpB) subunits of tryptophan synthase (Fig.
1). This is an unusual circumstance in
that most other organisms studied to date, both prokaryotic and
eukaryotic, have either the full complement of trp genes or
lack them altogether. A further heterogeneity within the
trpA gene of C. trachomatis has been identified
by Shaw et al. (22) in that serovar A and C appear to encode
a truncated version of TrpA compared with serovars D and L2 (22). The
differences in the trp gene complement among the chlamydiae characterized thus far suggest that the ability to synthesize tryptophan de novo is not required and raises the
possibility that these genes are in the process of being lost from the
genome. Alternatively, these differences may be important with respect to the unique tissue tropism of chlamydial strains and permit serovar-specific survival or growth within different microenvironments of the host.
(TrpB) subunits of tryptophan synthase (Fig.
1). This is an unusual circumstance in
that most other organisms studied to date, both prokaryotic and
eukaryotic, have either the full complement of trp genes or
lack them altogether. A further heterogeneity within the
trpA gene of C. trachomatis has been identified
by Shaw et al. (22) in that serovar A and C appear to encode
a truncated version of TrpA compared with serovars D and L2 (22). The
differences in the trp gene complement among the chlamydiae characterized thus far suggest that the ability to synthesize tryptophan de novo is not required and raises the
possibility that these genes are in the process of being lost from the
genome. Alternatively, these differences may be important with respect to the unique tissue tropism of chlamydial strains and permit serovar-specific survival or growth within different microenvironments of the host.
|
Tryptophan synthase is a tetramer consisting of two  subunits and
two
subunits and
two  subunits (23-25). This bifunctional enzyme catalyzes the two
final steps in the biosynthesis of tryptophan (Fig. 1), which are the
cleavage of indole glycerol 3-phosphate (IGP) to indole and
glyceraldehyde 3-phosphate (termed the
subunits (23-25). This bifunctional enzyme catalyzes the two
final steps in the biosynthesis of tryptophan (Fig. 1), which are the
cleavage of indole glycerol 3-phosphate (IGP) to indole and
glyceraldehyde 3-phosphate (termed the  reaction and catalyzed by
TrpA) followed by the
reaction and catalyzed by
TrpA) followed by the  -replacement reaction of indole with serine to
form tryptophan (the
-replacement reaction of indole with serine to
form tryptophan (the  reaction, catalyzed by TrpB). Extensive
characterization of the Escherichia coli and
Salmonella enzymes has demonstrated a large degree of
allosteric regulation and cooperativity between the
reaction, catalyzed by TrpB). Extensive
characterization of the Escherichia coli and
Salmonella enzymes has demonstrated a large degree of
allosteric regulation and cooperativity between the  and
and  subunits (23-25). In fact, TrpA and TrpB exhibit little activity in
their respective reactions in the absence of the other subunit
(26-28). Given the association of IFN-
subunits (23-25). In fact, TrpA and TrpB exhibit little activity in
their respective reactions in the absence of the other subunit
(26-28). Given the association of IFN-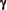 with chlamydial infections
and its effect on tryptophan levels in the host cell and, thus, on
chlamydial growth, encoding functional tryptophan synthase may be a
survival factor for intracellular chlamydiae. However, IGP substrate
for the
with chlamydial infections
and its effect on tryptophan levels in the host cell and, thus, on
chlamydial growth, encoding functional tryptophan synthase may be a
survival factor for intracellular chlamydiae. However, IGP substrate
for the  reaction in E. coli and Salmonella is
supplied by the sequential activity of the other genes of the
tryptophan biosynthesis pathway (TrpE, D, FC) (Fig. 1). C. trachomatis does not encode orthologues of TrpE, G, D, or C,
although paradoxically, it does have an orthologue of TrpF, the gene
for which lies outside the plasticity zone (18, 19). Because mammalian
cells lack the ability to biosynthesize tryptophan and C. trachomatis appears to lack the capability of IGP synthesis, it is
unclear what the substrate for chlamydial TrpA would be.
reaction in E. coli and Salmonella is
supplied by the sequential activity of the other genes of the
tryptophan biosynthesis pathway (TrpE, D, FC) (Fig. 1). C. trachomatis does not encode orthologues of TrpE, G, D, or C,
although paradoxically, it does have an orthologue of TrpF, the gene
for which lies outside the plasticity zone (18, 19). Because mammalian
cells lack the ability to biosynthesize tryptophan and C. trachomatis appears to lack the capability of IGP synthesis, it is
unclear what the substrate for chlamydial TrpA would be.
The present work involved a study of the diversity within the
trp region among all 15 C. trachomatis serovars
and characterization of the functionality of the tryptophan synthase
encoded therein. Here we report that all the C. trachomatis
type strain serovars, with the exception of B and MoPn, encode
homologues of trpB and trpA, that the gene
products are found in both EBs and RBs, and that the ability to
synthesize tryptophan differs among ocular and genital serovars.
Furthermore, we provide evidence that the  subunit of C. trachomatis tryptophan synthase differs from that of other
Gram-negative bacteria with respect to the utilization of IGP as a substrate.
subunit of C. trachomatis tryptophan synthase differs from that of other
Gram-negative bacteria with respect to the utilization of IGP as a substrate.
| |
MATERIALS AND METHODS |
|---|
|
|
|---|
Bacterial Strains, Plasmids, and Antibodies--
The bacterial
strains and plasmids utilized in this study are listed in Table
I. E. coli strains were grown
in Luria-Bertani (LB) broth or on LB agar and in the presence of 100 µg ml 1 ampicillin in the case of pQE-80L transformants.
C. trachomatis serovars were propagated in the HeLa 229 cervical carcinoma cell line (ATCC) maintained in minimal essential
medium (MEM, Invitrogen) supplemented with 10% heat-inactivated fetal
calf serum (FCS) as described previously (29). For growth of C. trachomatis under tryptophan-free conditions, dialyzed FCS was
used. C. trachomatis EBs were purified by density gradient
centrifugation according to established procedures and stored in
sucrose phosphate glycerol medium at
1 ampicillin in the case of pQE-80L transformants.
C. trachomatis serovars were propagated in the HeLa 229 cervical carcinoma cell line (ATCC) maintained in minimal essential
medium (MEM, Invitrogen) supplemented with 10% heat-inactivated fetal
calf serum (FCS) as described previously (29). For growth of C. trachomatis under tryptophan-free conditions, dialyzed FCS was
used. C. trachomatis EBs were purified by density gradient
centrifugation according to established procedures and stored in
sucrose phosphate glycerol medium at  80 °C (30). Polyclonal
antiserum against C. trachomatis TrpA was raised in rabbits
by immunization with purified recombinant serovar L2 TrpA. Mouse
ascites polyclonal anti-TrpB was raised against recombinant serovar L2
TrpB by following the procedure of Lacy and Voss (31).
80 °C (30). Polyclonal
antiserum against C. trachomatis TrpA was raised in rabbits
by immunization with purified recombinant serovar L2 TrpA. Mouse
ascites polyclonal anti-TrpB was raised against recombinant serovar L2
TrpB by following the procedure of Lacy and Voss (31).
|
Sequence Analysis of C. trachomatis trp Genes-- DNA between CT175 and CT167 of serovar D (18), containing the C. trachomatis trp genes, was amplified from chromosomal DNA of all 15 C. trachomatis serovars using primers 00.11 and JHC258 (Table II) and Expand High Fidelity polymerase according to manufacturer's instructions (Roche Molecular Biochemicals). After gel purification, the PCR products were cloned into pCR-XL-TOPO using the kit from Invitrogen, and the constructs were transformed into DH10B cells for propagation. Cloned insert DNA was sequenced by a commercial company (SeqWright, Houston, TX). The nucleotide (nt) and deduced amino acid sequences were aligned using ClustalW version 1.8. The following sequences have been submitted to GenBankTM: accession numbers AY096805 (serovar Atrp) AY096806 (Batrp), AY096807 (Ctrp), AY096808 (Dtrp), AY096809 (Etrp), AY096810 (Ftrp), Y096811 (Gtrp), AY096812 (Htrp), AY096813 (Itrp), AY096814 (Jtrp), AY096815 (Ktrp), AY096816 (L1trp), AY096817 (L2trp), and AY096818 (L3trp).
|
RT-PCR Analysis of trp Gene Expression--
Monolayers of HeLa
229 cells in T-175 flasks were infected with C. trachomatis
EBs at a multiplicity of infection (m.o.i) of 3-5 inclusion-forming
units (IFU) cell 1, as previously described (29). As a
negative control, a mock-infected flask was prepared in the same manner
but without the addition of EBs. The cells were incubated for 24 h
at 37 °C, and then total RNA was prepared using Trizol reagent
according to the manufacturer's instructions (Invitrogen). After
treatment with amplification-grade DNase I (Invitrogen), 1 µg of RNA
was reverse-transcribed using random hexamer primers and Thermoscript
reverse transcriptase (Invitrogen) and then treated with RNase H
(Invitrogen). Primers specific for 16 S rRNA and the
trpB-trpA junction (Table II) were used to amplify products
in PCR reaction mixtures containing 2 µl of cDNA, 0.2 µM primers, 0.2 mM dNTPs, 1.5 mM
MgCl2, 1× Taq reaction buffer, and 5 units of
Taq DNA polymerase (Invitrogen). The cycling program was 3 min at 95 °C followed by 30 cycles of 30 s at 95 °C, 30 s at 60 °C, and 1.5 min at 72 °C. Products were separated on a
1.5% agarose-Tris-buffered EDTA gel and visualized by ethidium
bromide staining.
1, as previously described (29). As a
negative control, a mock-infected flask was prepared in the same manner
but without the addition of EBs. The cells were incubated for 24 h
at 37 °C, and then total RNA was prepared using Trizol reagent
according to the manufacturer's instructions (Invitrogen). After
treatment with amplification-grade DNase I (Invitrogen), 1 µg of RNA
was reverse-transcribed using random hexamer primers and Thermoscript
reverse transcriptase (Invitrogen) and then treated with RNase H
(Invitrogen). Primers specific for 16 S rRNA and the
trpB-trpA junction (Table II) were used to amplify products
in PCR reaction mixtures containing 2 µl of cDNA, 0.2 µM primers, 0.2 mM dNTPs, 1.5 mM
MgCl2, 1× Taq reaction buffer, and 5 units of
Taq DNA polymerase (Invitrogen). The cycling program was 3 min at 95 °C followed by 30 cycles of 30 s at 95 °C, 30 s at 60 °C, and 1.5 min at 72 °C. Products were separated on a
1.5% agarose-Tris-buffered EDTA gel and visualized by ethidium
bromide staining.
Western Blot Analyses-- Purified EBs were lysed by suspension in Laemmli buffer followed by incubation at 95 °C for 10 min. Soluble proteins were fractionated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk and then incubated with anti-TrpA antiserum followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin or with anti-TrpB ascites followed by goat anti-mouse HRP. Bound antibodies were detected by enhanced chemiluminescence according to manufacturer's instructions (Amersham Biosciences).
Expression Cloning of trpB and trpA--
C.
trachomatis and E. coli trp genes were
amplified by PCR from purified chromosomal DNA using the reagent
concentrations described for RT-PCR and the cycling program 3 min at
95 °C followed by 30 cycles of 1 min at 95 °C, 30 s at
50 °C, and 2 min at 72 °C. The PCR primer sequences are listed in
Table II and were designed to include unique restriction sites for
cloning. For construction of plasmids to co-express trpB and
trpA, the 5'-CttrpB and the 3'-CttrpA
primers were used in the PCR reaction. The PCR products were
gel-purified, restricted with KpnI and SalI (for
C. trachomatis) or BamHI and KpnI (for
E. coli) and ligated to expression vector pQE-80L (Qiagen)
cut with the corresponding restriction enzymes. Constructs were
transformed into DH5 for screening, purified by miniprep, and then
used to transform E. coli mutant strains for complementation
assays. Constructs co-expressing serovar A trpB with serovar
L2 trpA and vice versa were prepared as follows. The
C. trachomatis trpA gene has a unique SpeI site
73 bp downstream of the start codon in a region of sequence identity
among all of the serovars. Plasmids pCFG6 (serovar A trpBA)
and pCR3 (serovar L2 trpBA) were restricted with
SpeI and KpnI, and the fragments were
gel-purified. The trpA-containing fragment from pCR3 was then ligated to the trpB-containing fragment from pCFG6 to
generate pCFG7. Similarly, pCFG8 was constructed by ligating the
trpA-containing fragment from pCFG6 to the
trpB-containing fragment from pCR3.
for screening, purified by miniprep, and then
used to transform E. coli mutant strains for complementation
assays. Constructs co-expressing serovar A trpB with serovar
L2 trpA and vice versa were prepared as follows. The
C. trachomatis trpA gene has a unique SpeI site
73 bp downstream of the start codon in a region of sequence identity
among all of the serovars. Plasmids pCFG6 (serovar A trpBA)
and pCR3 (serovar L2 trpBA) were restricted with
SpeI and KpnI, and the fragments were
gel-purified. The trpA-containing fragment from pCR3 was then ligated to the trpB-containing fragment from pCFG6 to
generate pCFG7. Similarly, pCFG8 was constructed by ligating the
trpA-containing fragment from pCFG6 to the
trpB-containing fragment from pCR3.
Complementation Assays--
The cells from stationary
phase cultures of E. coli trp transformants were harvested
by centrifugation and washed three times with sterile
phosphate-buffered saline. The cell suspensions were then streaked onto
minimal agar (1× M9 salts, 0.2% glucose, 0.2% casamino acids, 2 mM MgSO4, 0.2 mM
L-serine, 100 µg ml 1 ampicillin, and 50 µg ml
1 ampicillin, and 50 µg ml 1 each thiamine, cysteine, and uracil) containing
100 µM indole, 50 µg ml
1 each thiamine, cysteine, and uracil) containing
100 µM indole, 50 µg ml 1
L-tryptophan or without additional supplements. The plates
were incubated for 48 h at 37 °C and then photographed.
1
L-tryptophan or without additional supplements. The plates
were incubated for 48 h at 37 °C and then photographed.
Preparation of Cell Lysates for Enzyme Assays--
Five ml of
stationary phase cultures of CY15077 trp transformants were
used to inoculate 50 ml of LB broth containing 100 µg
ml 1 ampicillin. After incubation with aeration for 2 h at 37 °C, the cultures were cooled to 18 °C,
isopropyl-1-thio-
1 ampicillin. After incubation with aeration for 2 h at 37 °C, the cultures were cooled to 18 °C,
isopropyl-1-thio- -D-galactopyranoside (Invitrogen) was
added to a final concentration of 100 µM, and the
cultures were incubated with aeration for a further 18 h at 18 °C. The cells were then harvested by centrifugation, resuspended in 3 ml of 10 mM Tris-HCl, pH 7.8, and lysed by sonication
on ice. Cell debris was removed by centrifugation, and the cleared lysates were kept on ice. Protein concentration was determined by
Bradford assay using a commercial kit (Bio-Rad).
-D-galactopyranoside (Invitrogen) was
added to a final concentration of 100 µM, and the
cultures were incubated with aeration for a further 18 h at 18 °C. The cells were then harvested by centrifugation, resuspended in 3 ml of 10 mM Tris-HCl, pH 7.8, and lysed by sonication
on ice. Cell debris was removed by centrifugation, and the cleared lysates were kept on ice. Protein concentration was determined by
Bradford assay using a commercial kit (Bio-Rad).
Enzyme Assays--
One unit of activity is defined as the
appearance of 0.1 µmol of product ( reaction) or the disappearance
of 0.1 µM substrate (
reaction) or the disappearance
of 0.1 µM substrate ( and
and 
 reactions) in 20 min
at 37 °C. The
reactions) in 20 min
at 37 °C. The  reaction assays and the
reaction assays and the 
 reaction assays
were performed using the methods of Smith and Yanofsky (32). The
reaction assays
were performed using the methods of Smith and Yanofsky (32). The  reaction mixture contained 0.3 µmol of IGP, 100 µmol of phosphate
buffer, pH 7.0, 2 µmol of NH2OH, and 70 µl of cell
lysate in a final volume of 0.5 ml. The
reaction mixture contained 0.3 µmol of IGP, 100 µmol of phosphate
buffer, pH 7.0, 2 µmol of NH2OH, and 70 µl of cell
lysate in a final volume of 0.5 ml. The 
 reaction mixture
contained 0.4 µmol of IGP, 80 µmol of L-serine, 0.03 µmol of pyridoxal phosphate, 100 µmol of Tris buffer, pH 7.8, 30 µl of saturated NaCl, and 70 µl of cell lysate in a final volume of
1 ml. The
reaction mixture
contained 0.4 µmol of IGP, 80 µmol of L-serine, 0.03 µmol of pyridoxal phosphate, 100 µmol of Tris buffer, pH 7.8, 30 µl of saturated NaCl, and 70 µl of cell lysate in a final volume of
1 ml. The  reaction assays were performed using the method of Miles
(33). The
reaction assays were performed using the method of Miles
(33). The  reaction mixture contained 0.1 µmol of indole, 20 µmol of L-serine, 0.0075 µmol of pyridoxal phosphate, 25 µmol of Tris buffer, pH 7.8, 7.5 µl of saturated NaCl, and 50 µl of cell lysate in a final volume of 250 µl. Specific activity (units mg
reaction mixture contained 0.1 µmol of indole, 20 µmol of L-serine, 0.0075 µmol of pyridoxal phosphate, 25 µmol of Tris buffer, pH 7.8, 7.5 µl of saturated NaCl, and 50 µl of cell lysate in a final volume of 250 µl. Specific activity (units mg 1) is reported as the average of triplicate determinations.
1) is reported as the average of triplicate determinations.
C. trachomatis Growth Assays--
Monolayers of HeLa 229 cells
in 6-well plates were infected with C. trachomatis EBs at an
m.o.i. of 3-5 IFU cell 1 in MEM plus 10% dialyzed fetal
bovine serum supplemented with tryptophan (10 mg liter
1 in MEM plus 10% dialyzed fetal
bovine serum supplemented with tryptophan (10 mg liter 1)
lacking tryptophan or lacking tryptophan but supplemented with indole
(100 µM) or varying concentrations of anthranilate. For tryptophan-free conditions, HeLa cells were incubated for 6 h in
tryptophan-free MEM plus 10% dialyzed fetal bovine serum before infection with C. trachomatis to ensure depletion of
endogenous tryptophan. Infected monolayers were incubated at 37 °C
for 48 h (LGV serovars) or 72 h (genital and ocular
serovars), after which time the medium was collected, and the cells
were lysed in cold, distilled water. Aliquots of the combined HeLa cell
lysates and culture medium were used to infect fresh HeLa cell
monolayers. Recoverable IFU were enumerated as previously described
(30).
1)
lacking tryptophan or lacking tryptophan but supplemented with indole
(100 µM) or varying concentrations of anthranilate. For tryptophan-free conditions, HeLa cells were incubated for 6 h in
tryptophan-free MEM plus 10% dialyzed fetal bovine serum before infection with C. trachomatis to ensure depletion of
endogenous tryptophan. Infected monolayers were incubated at 37 °C
for 48 h (LGV serovars) or 72 h (genital and ocular
serovars), after which time the medium was collected, and the cells
were lysed in cold, distilled water. Aliquots of the combined HeLa cell
lysates and culture medium were used to infect fresh HeLa cell
monolayers. Recoverable IFU were enumerated as previously described
(30).
Indole Incorporation Assays--
HeLa cell monolayers in
6-well plates were infected with C. trachomatis EBs at an
m.o.i. of 3-5 IFU cell 1 in the absence or presence of
tryptophan (10 mg liter
1 in the absence or presence of
tryptophan (10 mg liter 1) in MEM plus 10% dialyzed fetal
bovine serum supplemented with 100 µM
[14C]indole (0.1 µCi µM
1) in MEM plus 10% dialyzed fetal
bovine serum supplemented with 100 µM
[14C]indole (0.1 µCi µM 1).
Where indicated, the cells were incubated for 6 h in
tryptophan-free MEM plus 10% dialyzed fetal bovine serum before
infection. After incubation for 36 h (serovar L2) or 48 h
(serovars A, D, and I) at 37 °C, the medium was removed, the cells
were washed with Hanks' buffered saline solution, and then the cells
were lysed in cold, distilled water. Proteins in the cell lysate were
precipitated with 10% trichloroacetic acid, and incorporated
14C was quantified by scintillation counting (Beckman LS
5000). Data are expressed as dpm incorporated per 104 cells.
1).
Where indicated, the cells were incubated for 6 h in
tryptophan-free MEM plus 10% dialyzed fetal bovine serum before
infection. After incubation for 36 h (serovar L2) or 48 h
(serovars A, D, and I) at 37 °C, the medium was removed, the cells
were washed with Hanks' buffered saline solution, and then the cells
were lysed in cold, distilled water. Proteins in the cell lysate were
precipitated with 10% trichloroacetic acid, and incorporated
14C was quantified by scintillation counting (Beckman LS
5000). Data are expressed as dpm incorporated per 104 cells.
| |
RESULTS |
|---|
|
|
|---|
Sequence Analysis of trp Genes from C. trachomatis Ocular and
Genital Serovars--
Chlamydiae sequence data are available for both
tryptophan synthase subunits from the serovar D genome sequencing
project (18). Serovar D TrpB contains 392 amino acids, giving a
calculated molecular mass of 42.6 kDa, similar to the E. coli TrpB (34). A comparison of the complete amino acid sequence
of the serovar D TrpB with that of representative TrpBs in the public
databases (Fig. 2A) indicates
that the proteins are ~54% identical. Most importantly amino acid
residues identified as essential for enzyme activity, indole binding,
and pyridoxal phosphate-Lys87 Schiff base complex formation
in E. coli TrpB (His86, Lys87,
Glu109, Arg148, Leu188,
Cys230, Asp305, Phe306,
Glu350) (25, 33, 35-39) are conserved in serovar D
TrpB.
|
Serovar D TrpA protein contains 253 amino acids, a size similar to that of E. coli TrpA (40). A comparison of the complete amino acid sequence of serovar D TrpA with that of representative TrpAs in the public databases shows that the overall level of homology is low (27% identity, Fig. 2B). The two amino acids identified as essential for catalytic activity Glu49and Asp60 (41-43), are conserved in serovar D TrpA. Surprisingly several amino acids which form the active site pocket and/or have been identified by mutagenesis as essential for TrpA activity in E. coli (Phe22, Thr183, Gly211, Gly213, Gly234, Ser235) (23-25, 39, 44-47) are not conserved in serovar D TrpA. Interestingly, of all the TrpA sequences deposited in the public databases, only the chlamydial TrpAs show these amino acid changes in the active site.
The unusual primary structure of the serovar D TrpA and the previously reported truncation of TrpA in serovars A and C (22) prompted us to extend our investigations on tryptophan synthase to all human C. trachomatis serovars. We sequenced the trpB and trpA genes from the laboratory-type strains of all 15 C. trachomatis serovars to determine the diversity within this region. Consistent with the results of Shaw et al. (22), we were unable to amplify products from serovar B chromosomal DNA, indicating a deletion of the trp region from this isolate. The sequences of the trpB genes from the 14 serovars are remarkably similar, with only 6 single nucleotide polymorphisms present in 1179 nt (data not shown). One point mutation (nt 1017) does not alter the amino acid sequence (Asn339), a second point mutation (nt 206) is conservative, resulting in a change from Arg to Lys at position 69 in serovars H, J, L2, and L3, a third point mutation (nt 696) results in a change from Ser to Phe at position 232 in serovar L1, and a fourth point mutation (nt 1143) results in a change from Pro to Ser at position 381 in serovar Ba. The remaining two point mutations at nt 107 and 179 result in the conversion of Ser36 to Asn in serovars H, J, L2, and L3 and Asn60 to Ser in serovars A and C, respectively.
Examination of the nucleotide alignment of the 14 C. trachomatis trpA sequences revealed both single
nucleotide polymorphisms and deletion mutations in the 762-nt gene (all
numbering is based on genital serovar sequences unless otherwise
noted). Of the 11 point mutations identified, 4 (nt 10, 120, 477, and
699) are silent, 2 (nt 110 and 344) result in conservative amino acid
changes, Gln 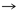 Arg and Ala
Arg and Ala 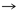 Val, in serovars H, J, L2, L3, and
three (nt 39) result in changes, Leu
Val, in serovars H, J, L2, L3, and
three (nt 39) result in changes, Leu 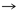 Pro in serovars D and K (data
not shown). The remaining mutations illustrated in Fig.
3 are more likely to have effects on
enzyme structure and/or function. The point mutations at nt 499 and 511 result in non-conservative amino acid substitutions and cluster the
serovars into two groups. Thus, all of the ocular serovars encode
His167 and Leu171, whereas all of the genital
serovars encode Tyr and Phe at the corresponding positions. The ocular
and genital serovars also differ in sequence at nt 408-410; these nt
are deleted in the ocular serovars, resulting in the loss of
Phe136 from the protein encoded by these genes (Fig. 3).
The trpA genes of the ocular serovars also have a single nt
deletion at position 528 (ocular serovar numbering) that results in a
frameshift generating a putative stop codon at nt 550-552 (ocular
serovar numbering). These deletion mutations were previously reported
for serovars A and C (22); here we demonstrate that they are also found
in serovar Ba. The deletion mutation found at nt 528 in the ocular serovars lies within a mutation "hotspot" for trpA.
Thus, in the genital serovars there are two point mutations found
within this same region at nt 530 and 532 encompassing two codons at
amino acid positions 177 and 178. The net result of these mutations is
that all LGV serovars encode Tyr177Glu178,
serovars D, K, and E encode Cys177-Gln178, and
serovars G, F, I, H, and J encode Tyr177-Gln178
(Fig. 3).
Pro in serovars D and K (data
not shown). The remaining mutations illustrated in Fig.
3 are more likely to have effects on
enzyme structure and/or function. The point mutations at nt 499 and 511 result in non-conservative amino acid substitutions and cluster the
serovars into two groups. Thus, all of the ocular serovars encode
His167 and Leu171, whereas all of the genital
serovars encode Tyr and Phe at the corresponding positions. The ocular
and genital serovars also differ in sequence at nt 408-410; these nt
are deleted in the ocular serovars, resulting in the loss of
Phe136 from the protein encoded by these genes (Fig. 3).
The trpA genes of the ocular serovars also have a single nt
deletion at position 528 (ocular serovar numbering) that results in a
frameshift generating a putative stop codon at nt 550-552 (ocular
serovar numbering). These deletion mutations were previously reported
for serovars A and C (22); here we demonstrate that they are also found
in serovar Ba. The deletion mutation found at nt 528 in the ocular serovars lies within a mutation "hotspot" for trpA.
Thus, in the genital serovars there are two point mutations found
within this same region at nt 530 and 532 encompassing two codons at
amino acid positions 177 and 178. The net result of these mutations is
that all LGV serovars encode Tyr177Glu178,
serovars D, K, and E encode Cys177-Gln178, and
serovars G, F, I, H, and J encode Tyr177-Gln178
(Fig. 3).
|
Expression of trp Genes during HeLa Cell Infection and in Purified
EBs--
To determine whether the trpB and trpA
genes were expressed in the various C. trachomatis serovars,
RT-PCR and Western blot analyses were carried out on type strains
representative of the LGV (L2), ocular (A), and genital serovars (D and
I). For RT-PCR analysis, RNA was prepared from mid-phase (24 h)
infected HeLa cell cultures. After reverse transcription, the cDNA
was amplified using a forward primer complementary to the 3' end of
trpB and a reverse primer complementary to the 5' end of
trpA. PCR products of the expected size were amplified from
cDNA derived from HeLa cells infected with serovars L2, A, I, and D
as well as from a plasmid (pCR3) containing full-length L2
trpB and trpA (Fig.
4A). These primers were
specific for C. trachomatis-derived mRNA, as no product
was amplified from cDNA prepared from mock-infected HeLa cells.
Similarly, primers specific for C. trachomatis 16 S rRNA
only amplified products from C. trachomatis-infected HeLa cell cDNA and a plasmid control but not the mock-infected sample. These data indicate that type strains representative of the serovars causing human disease all express trpB and trpA
and that these genes are transcribed as an operon. Furthermore, the
single base deletion mutation found in serovar A trpA does
not appear to affect transcription of trpBA mRNA.
|
Western blot analyses were used to determine whether the trp gene messages expressed by serovars L2, A, I, and D were translated to protein products. As shown in Fig. 4B, immunoreactive material of the same electrophoretic mobility as recombinant L2 TrpB was detected in purified EBs from serovars L2, A, I, and D. Similarly, material with the same mobility as recombinant L2 TrpA was detected in EB lysates of serovars L2, I, and D. Serovar A EBs also had anti-TrpA immunoreactive material but of lower molecular weight than that of the other serovars. This result indicates that the frameshift mutation in serovar A trpA results in the production of a truncated version of TrpA, consistent with the observations of Shaw et al. (22). No immunoreactive material of the appropriate size for either TrpB or TrpA was detectable in EBs from serovar B or biovar MoPn (data not shown), consistent with our inability to amplify products from these isolates using trpB- or trpA-specific primers (data not shown) and the absence of trpBA genes in the MoPn genome (19).
Genetic Complementation and in Vitro Enzyme Assays--
To
determine whether the TrpB and TrpA proteins expressed by C. trachomatis were catalytically active, a heterologous
complementation system was utilized. The trp genes from
serovars L2, A, and I were cloned into an E. coli expression
vector and transformed into E. coli mutants lacking various
components of the tryptophan biosynthesis pathway. The ability of the
E. coli mutants expressing C. trachomatis
trp genes to grow on minimal medium was then assessed (Fig.
5). The E. coli mutant KS463
expresses a non-functional TrpA but expresses active TrpB. KS463 cells
transformed with either expression vector alone or constructs
expressing C. trachomatis (serovars L2, A, or I) or E. coli trpA were able to grow on minimal medium supplemented with
indole. These data are consistent with published observations
indicating that E. coli TrpB can utilize indole in the
absence of functional TrpA (26-28). Similarly, all KS463 transformants
were able to grow on minimal medium supplemented with tryptophan, as
expected. However, KS463 failed to grow on minimal medium when
transformed with either the expression vector alone or any of the
constructs expressing C. trachomatis trpA regardless of the serovar of origin. In contrast, KS463 transformed with the E. coli trpA construct did grow on minimal medium.
These data suggested that C. trachomatis TrpA could not
efficiently utilize the IGP produced by KS463, either due to a loss of
catalytic activity for this substrate or due to an inability to
interact with and, thus, be activated by E. coli TrpB.
|
To distinguish between these two possibilities, the E. coli trpB transposon mutant BW7622, which does not express trpB or trpA, was transformed with constructs co-expressing C. trachomatis trpB and trpA. This eliminated the requirement for C. trachomatis TrpA to interact with a heterologous TrpB. There was no detectable growth of any of the transformants on minimal medium, whereas expression of tryptophan synthase derived from serovars L2 and I complemented the growth of BW7622 on indole-supplemented media (Fig. 5). These data suggest that C. trachomatis tryptophan synthase is unable to utilize IGP or does so at levels insufficient to complement growth of BW7622. Furthermore, efficient utilization of indole by C. trachomatis TrpB appeared to require the presence of full-length TrpA since serovar A tryptophan synthase expression did not rescue the growth of BW7622 on indole, although this transformant was able to grow on tryptophan-supplemented media. This conclusion was confirmed by complementation experiments carried out in an E. coli deletion mutant, CY15077, that lacks the entire tryptophan biosynthesis operon. Thus, transformation of CY15077 with constructs expressing only C. trachomatis trpB did not complement growth on minimal medium supplemented with indole. However, co-expression of trpB and trpA from serovars L2 and I did rescue the growth of CY15077 on indole-supplemented media. Similar to the results observed with BW7622, co-expression of serovar A trpB and trpA did not allow for growth of CY15077 on indole. This was likely due to the inability of serovar A TrpA to activate serovar A TrpB, since the co-expression of serovar A TrpB with serovar L2 TrpA permitted the growth of CY15077 on indole. In contrast, expression of serovar L2 TrpB with serovar A TrpA failed to rescue the growth of CY15077 on indole.
In addition to the genetic complementation studies, in vitro
activity in the  ,
,  , and
, and 
 reactions was determined for
cellular extracts prepared from E. coli CY15077
co-expressing C. trachomatis trpB and trpA (Table
III). As positive control, activity in
all three reactions was detected for purified tryptophan synthase from
Salmonella enterica ser. Typhimurium. No activity was
detected in any of the assays using cellular extracts from CY15077
cells transformed with the expression vector alone. As expected from the results of the complementation studies, no activity for the
reactions was determined for
cellular extracts prepared from E. coli CY15077
co-expressing C. trachomatis trpB and trpA (Table
III). As positive control, activity in
all three reactions was detected for purified tryptophan synthase from
Salmonella enterica ser. Typhimurium. No activity was
detected in any of the assays using cellular extracts from CY15077
cells transformed with the expression vector alone. As expected from the results of the complementation studies, no activity for the  or
or

 reactions was detectable in any of the lysates of cells expressing C. trachomatis proteins. In contrast, activity
for the
reactions was detectable in any of the lysates of cells expressing C. trachomatis proteins. In contrast, activity
for the  reaction was readily detectable in lysates containing TrpB and TrpA from serovars L2 and I, whereas the
reaction was readily detectable in lysates containing TrpB and TrpA from serovars L2 and I, whereas the  reaction activity of
the lysate of serovar A-expressing cells was very low. Taken together,
results from the complementation studies and in vitro enzyme
assays suggest that unlike the Salmonella and E. coli enzymes, tryptophan synthase from C. trachomatis
is unable to efficiently catalyze the conversion of IGP to indole (
reaction activity of
the lysate of serovar A-expressing cells was very low. Taken together,
results from the complementation studies and in vitro enzyme
assays suggest that unlike the Salmonella and E. coli enzymes, tryptophan synthase from C. trachomatis
is unable to efficiently catalyze the conversion of IGP to indole ( reaction) and reaction of indole with serine to form tryptophan (
reaction) and reaction of indole with serine to form tryptophan ( reaction) requires the presence of a full-length TrpA. Because the
specific activity in the
reaction) requires the presence of a full-length TrpA. Because the
specific activity in the  reaction for L2 and I extracts was low
compared with the purified Salmonella enzyme, further
studies with purified C. trachomatis enzymes will be
required to confirm whether there is indeed no
reaction for L2 and I extracts was low
compared with the purified Salmonella enzyme, further
studies with purified C. trachomatis enzymes will be
required to confirm whether there is indeed no  activity or whether
it is just too low to be detected in cellular extracts.
activity or whether
it is just too low to be detected in cellular extracts.
|
C. trachomatis Tryptophan Synthase Activity in Vivo--
It is
clear from the complementation and in vitro activity studies
that the trpB genes of C. trachomatis serovars
L2, A, and I encode functional enzymes that require the presence of
full-length TrpA for detectable activity. It has been previously
reported that growth of most human serovars of C. trachomatis are tryptophan-dependent (48-50). To
determine whether C. trachomatis tryptophan synthase could
function in vivo, HeLa cell infections were carried out under tryptophan-free conditions, and chlamydial growth was assessed after supplementation of the media with potential tryptophan
precursors. It has been proposed that anthranilate may serve as a
precursor for tryptophan biosynthesis in C. trachomatis
(51). However, the decrease in recoverable IFU for serovar L2 grown in
tryptophan-free media did not change after supplementation with
anthranilate (Fig. 6). Thus, anthranilate
cannot by used by C. trachomatis for tryptophan synthesis.
In addition, kynurenine, another tryptophan degradation product, was
also unable to rescue C. trachomatis growth in
tryptophan-efficient medium (data not shown).
|
Results from the in vitro enzyme assays indicated that
C. trachomatis TrpB could utilize indole for the synthesis
of tryptophan. As shown in Fig. 7, the
level of recoverable IFU under tryptophan-replete conditions varied
depending upon the serovar. However, growth of all serovars was
inhibited by the removal of tryptophan from the cell culture medium. In
the presence of 100 µM indole, growth of all the genital
(D-K, L1-L3) serovars recovered to tryptophan-replete levels or better,
whereas there was no effect of indole supplementation on the growth of
the ocular serovars (A-C, Ba). To confirm that indole was being
utilized by C. trachomatis and was not converted into a
tryptophan precursor by the host cell, HeLa cell infections were
carried out in the presence of radiolabeled indole. All C. trachomatis serovars grown in the presence of tryptophan failed to
incorporate 14C-labeled indole, suggesting that there may
be some regulation of tryptophan synthase by tryptophan levels in the
cell. Under tryptophan-free conditions, serovars L2, D, and I were able
to incorporate 14C-labeled indole, whereas serovar A and
mock-infected HeLa cells showed no indole incorporation (Fig.
8). Thus, it would appear that in
vivo, C. trachomatis genital serovars are able to
synthesize tryptophan directly from indole and that lack of a
full-length TrpA results in the inability of ocular serovars to utilize
indole.
|
|
| |
DISCUSSION |
|---|
|
|
|---|
Host response to chlamydial infection involves production of the
protective cytokine IFN-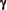 , which induces the expression of indoleamine-2,3-dioxygenase and, thus, promotes tryptophan degradation in the host cells (14, 15, 52). Thus, the ability to synthesize tryptophan may be an important survival factor for C. trachomatis during the course of infection, allowing the
intracellular bacteria to persist in the presence of IFN-
, which induces the expression of indoleamine-2,3-dioxygenase and, thus, promotes tryptophan degradation in the host cells (14, 15, 52). Thus, the ability to synthesize tryptophan may be an important survival factor for C. trachomatis during the course of infection, allowing the
intracellular bacteria to persist in the presence of IFN- -induced
tryptophan limitation. This study was undertaken to determine the
extent of heterogeneity in the C. trachomatis trp
region and to determine whether trpB and trpA,
respectively, encoding the
-induced
tryptophan limitation. This study was undertaken to determine the
extent of heterogeneity in the C. trachomatis trp
region and to determine whether trpB and trpA,
respectively, encoding the  and
and  subunits of tryptophan synthase,
were expressed as functional enzymes.
subunits of tryptophan synthase,
were expressed as functional enzymes.
Both trpB and trpA were expressed in C. trachomatis as shown by RT-PCR analysis of transcripts from infected HeLa cells. Results indicate that trpB and trpA can be expressed as a single transcript, similar to what has been observed for the trp operon of other bacteria (53, 54). C. trachomatis encodes a putative tryptophan repressor (trpR), which presents the possibility that transcription of the trp genes may be regulated by the tryptophan concentration in the host cell. High levels transcriptional repression of the trp operon by the TrpR-tryptophan repressor complex has been observed in many Gram-negative bacteria (55). However, results from the present study indicate that there must be a basal level of trp gene expression in C. trachomatis, as we could detect both TrpB and TrpA in purified EBs obtained from HeLa cells infected in tryptophan-replete medium.
The C. trachomatis trpB gene sequences from the
15 reference serovars were nearly identical, with only four single
nucleotide polymorphisms observed. All of the amino acids essential for
activity, as identified in E. coli TrpB (His86,
Lys87, Glu109, Arg148,
Leu188, Cys230, Asp305,
Phe306, Glu350) (33, 35-38) are conserved in
the C. trachomatis proteins, suggesting that they should
have enzymatic activity. This was indeed the case as shown by both
genetic complementation studies and in vitro assays of  reaction activity in crude cell lysates. TrpB from serovars L2, A, and
I was capable of catalyzing the
reaction activity in crude cell lysates. TrpB from serovars L2, A, and
I was capable of catalyzing the  -replacement reaction of indole and
serine to form tryptophan. However, our results suggested a unique
property of the C. trachomatis TrpB compared with that
characterized from other Gram-negative bacteria. Thus, C. trachomatis TrpB appeared to have an absolute requirement for TrpA
for function; no
-replacement reaction of indole and
serine to form tryptophan. However, our results suggested a unique
property of the C. trachomatis TrpB compared with that
characterized from other Gram-negative bacteria. Thus, C. trachomatis TrpB appeared to have an absolute requirement for TrpA
for function; no  reaction activity was detectable in the absence of
TrpA or in the presence of truncated TrpA from serovar A. In contrast,
E. coli TrpB has been shown to have activity in the absence
of TrpA, albeit at a lower level than observed in its presence
(26-28). Therefore, the requirement for TrpA activation of TrpB
appears to be more stringent in the C. trachomatis enzyme than in that of E. coli or Salmonella.
reaction activity was detectable in the absence of
TrpA or in the presence of truncated TrpA from serovar A. In contrast,
E. coli TrpB has been shown to have activity in the absence
of TrpA, albeit at a lower level than observed in its presence
(26-28). Therefore, the requirement for TrpA activation of TrpB
appears to be more stringent in the C. trachomatis enzyme than in that of E. coli or Salmonella.
A larger number of polymorphisms were found in the nucleotide sequences
of C. trachomatis trpA compared with
trpB; however, there was still greater than 98% identity
among the sequences from the various serovars. Interestingly, TrpA from
all serovars retained the invariant catalytic residues,
Glu49 and Asp60, but had changed most of the
other highly conserved amino acids (Phe22,
Thr183, Gly211, Gly213,
Gly234, Ser235) in the active site pocket.
These residues have been shown by mutagenesis to be critical for TrpA
activity (41, 42, 44-46, 56) and are key residues involved in binding
IGP in the Salmonella TrpBA crystal structure (25, 39, 47).
Given the changes in these key amino acids, it is not surprising that
we did not detect  reaction activity in the genetic complementation
studies nor the in vitro activity assays of lysates from
cells overexpressing the C. trachomatis tryptophan synthase.
reaction activity in the genetic complementation
studies nor the in vitro activity assays of lysates from
cells overexpressing the C. trachomatis tryptophan synthase.
A polymorphic mutational "hot spot" was identified in
trpA from the genital chlamydiae serovars at nt 530 and 532, resulting in three possible amino acids combinations at positions 177 and 178 in the translated protein. These amino acids lie in TrpA loop 6, a region identified in the Salmonella tryptophan synthase
crystal structure as being highly flexible and important for
subunit-subunit interactions between TrpB and TrpA, metabolite
channeling, and substrate binding (23, 24, 39, 57-61). It is possible
that the sequence polymorphisms observed in the TrpA loop 6 region may
affect interactions between the  and
and  subunits and thus influence
TrpB activity. In total, the unusual primary structure of TrpA suggests
that the main function of the C. trachomatis
subunits and thus influence
TrpB activity. In total, the unusual primary structure of TrpA suggests
that the main function of the C. trachomatis  -subunit
could be to position TrpB in the appropriate or favorable conformation
to efficiently carry out the
-subunit
could be to position TrpB in the appropriate or favorable conformation
to efficiently carry out the  reaction. Detailed kinetic
characterization of purified TrpB and TrpA from the different serovars
will be required to determine this.
reaction. Detailed kinetic
characterization of purified TrpB and TrpA from the different serovars
will be required to determine this.
The deletion and frameshift mutations in trpA from serovars A and C, originally identified by Shaw et al. (22), were also found in serovar Ba in the present study. Interestingly, these mutations appear to be predictive for the tissue tropism of the isolates. All of the ocular serovars had a 3-base deletion (nt 408-410) and a single nucleotide deletion (nt 528), resulting in a truncated TrpA, whereas none of the genital serovars did. Similarly, non-conservative point mutations also cluster the serovars into ocular and genital strains (i.e. at nt 499 and 511). We are currently investigating whether this correlation between trpA sequence and serovar tissue tropism holds true for clinical isolates.
It has been postulated that C. trachomatis may be able to scavenge tryptophan degradation products such as anthranilate from the host cell for use as precursors in tryptophan biosynthesis (51). Results from the present study clearly indicate that anthranilate could not rescue C. trachomatis growth in HeLa cells grown in tryptophan-free medium. This is not surprising given the absence of several key enzymes in the tryptophan biosynthesis pathway. Although C. trachomatis encodes a phosphoribosyl anthranilate isomerase (trpF), it lacks the gene for anthranilate phosphoribosyltransferase required for conversion of anthranilate to phosphoribosyl anthranilate as well as the gene for IGP synthase (trpC), required for the conversion of 1-(o-carboxylphenylamino)-1-deoxyribulose-5-phosphate to IGP (see Fig. 1).
Because of the lack of tools for genetic manipulation, it has not been
possible to produce site-specific mutants in C. trachomatis. Despite this limitation, our results indicate that the tryptophan synthase detected in C. trachomatis RBs appears to function
similarly to the recombinant enzymes expressed in E. coli.
Thus, C. trachomatis growing within HeLa cells was able to
utilize indole for growth in the absence of tryptophan. Only genital
serovars could utilize indole, consistent with the observations that
in vitro the truncated TrpA (found in ocular serovars) could
not enhance TrpB activity in the  reaction. Indole was used directly
by C. trachomatis and was not processed by the HeLa cells
into some other precursor molecule, as mock-infected cells exhibited no
[14C]indole incorporation. In addition, C. trachomatis serovar A could not incorporate
]14C]indole, further confirming that its TrpB is unable
to function in the absence of full-length TrpA.
reaction. Indole was used directly
by C. trachomatis and was not processed by the HeLa cells
into some other precursor molecule, as mock-infected cells exhibited no
[14C]indole incorporation. In addition, C. trachomatis serovar A could not incorporate
]14C]indole, further confirming that its TrpB is unable
to function in the absence of full-length TrpA.
Taken together, our results indicate that tryptophan synthase encoded by C. trachomatis trpBA is functional for conversion of indole to tryptophan, which permits the growth of genital serovars under conditions of tryptophan starvation. In stark contrast, ocular serovars A, Ba, and C, with a mutation in trpA, resulting in the production of a truncated protein, are unable to utilize indole for growth in the absence of tryptophan. What might the clinical significance of these findings be? It is well known that ocular serovars rarely cause genital infections, and genital serovars are rarely associated with blinding trachoma (2, 3, 9). The molecular basis for this distinct tissue tropism has never been defined. To our knowledge the differing abilities to synthesize tryptophan is the first demonstration of a distinction in the biosynthetic capacity between the ocular and genital serovars.
The unusual properties of the chlamydial tryptophan synthase raise the
question as to what might be the true substrate in vivo. Our
findings support the hypothesis that it is likely indole. Under normal
physiological conditions, indole is not readily available as a
metabolite in mammalian cells. Indole is, however, a major byproduct of
tryptophan degradation in bacteria encoding the enzyme tryptophanase
(62). Common enteric bacteria such as E. coli and
Proteus sp. are known producers of indole (62, 63). The same
two organisms are also part of the normal genital microflora and
important urogenital pathogens (64, 65). Other indole-producing organisms (66) known to colonize the female genital tract (65) include
Peptostreptococcus asaccharolyticus,
Fusobacterium species, Bacteroides species,
Haemophilus influenza, and Weeksella virosa. This
raises the intriguing possibility that C. trachomatis
serovars that infect the genital tract may be able to use indole
produced by other microflora, either endogenous or the result of
infection, as a substrate to synthesize their own tryptophan for
growth. Co-infection with indole-producing organisms may allow for the rescue of chlamydial organisms persisting in a non-replicating form in
response to host IFN- and its subsequent effect on intracellular tryptophan levels. The ability to synthesize tryptophan from indole may
be important for the persistence of C. trachomatis within the genital tract epithelium, with important consequences for disease
transmission as well as for the inflammatory sequelae associated with
chronic infection. Because the eye is normally a sterile niche, ocular
serovars are less likely to encounter sources of indole during the
course of infection, thereby eliminating the selective pressure to
maintain a functional tryptophan synthase.
and its subsequent effect on intracellular tryptophan levels. The ability to synthesize tryptophan from indole may
be important for the persistence of C. trachomatis within the genital tract epithelium, with important consequences for disease
transmission as well as for the inflammatory sequelae associated with
chronic infection. Because the eye is normally a sterile niche, ocular
serovars are less likely to encounter sources of indole during the
course of infection, thereby eliminating the selective pressure to
maintain a functional tryptophan synthase.
| |
ACKNOWLEDGEMENTS |
|---|
We thank Dr. Edith Wilson Miles for providing us with purified Salmonella tryptophan synthase and IGP and Dr. Charles Yanofsky for several E. coli mutants. We are also indebted to both for helpful advice.
| |
FOOTNOTES |
|---|
* This work was supported by research Grant GR-13301 from the Canadian Institutes of Health Research (to G. M.) and a postdoctoral fellowship from the Manitoba Health Research Council (to C. F. G.).The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) AY096805 (serovar Atrp) AY096806 (Batrp), AY096807 (Ctrp), AY096808 (Dtrp), AY096809 (Etrp), AY096810 (Ftrp), Y096811 (Gtrp), AY096812 (Htrp), AY096813 (Itrp), AY096814 (Jtrp), AY096815 (Ktrp), AY096816 (L1trp), AY096817 (L2trp), and AY096818 (L3trp).
 To whom correspondence should be addressed: Dept. of Medical
Microbiology, University of Manitoba, Winnipeg, Manitoba R3E 0W3,
Canada. Tel.: 204-789-3307; Fax: 204-789-3926; E-mail:
mcclart@cc.umanitoba.ca.
To whom correspondence should be addressed: Dept. of Medical
Microbiology, University of Manitoba, Winnipeg, Manitoba R3E 0W3,
Canada. Tel.: 204-789-3307; Fax: 204-789-3926; E-mail:
mcclart@cc.umanitoba.ca.
Published, JBC Papers in Press, May 13, 2002, DOI 10.1074/jbc.M203937200
| |
ABBREVIATIONS |
|---|
The abbreviations used are: EB, elementary body; RB, reticulate body; LGV, lymphogranuloma venereum; IFN, interferon; IGP, indole glycerol 3-phosphate; MEM, minimal essential medium; FCS, fetal calf serum; nt, nucleotide(s); RT, reverse transcription; IFU, inclusion-forming units.
| |
REFERENCES |
|---|
|
|
|---|
| 1. | Moulder, J. W. (1991) Microbiol. Rev. 55, 143-190 |
| 2. | Schachter, J. (1989) Pathol. Immunopathol. Res. 8, 206-220[Medline] [Order article via Infotrieve] |
| 3. | Schachter, J. (1999) in Chlamydia Intracellular Biology, Pathogenesis, and Immunity (Stephens, R. S., ed) , pp. 139-170, American Society for Microbiology, Washington, D. C. |
| 4. | Kuo, C. C., Jackson, L. A., Campbell, L. A., and Grayston, J. T. (1995) Clin. Microbiol. Rev. 8, 451-461[Abstract] |
| 5. | Campbell, L. A., Rosenfeld, M., and Kuo, C. C. (2000) Trends Microbiol. 8, 255-257[CrossRef][Medline] [Order article via Infotrieve] |
| 6. | Kuo, C., and Campbell, L. A. (1998) Mol. Med. Today 4, 426-430[CrossRef][Medline] [Order article via Infotrieve] |
| 7. | Grayston, J. T., Kuo, C. C., Campbell, L. A., Wang, S. P., and Jackson, L. A. (1997) Cardiologia 42, 1145-1151[Medline] [Order article via Infotrieve] |
| 8. | Stephens, R. (1989) in Antigenic Variation of Chlamydia trachomatis (Moulder, J.W., ed) , CRC Press, Inc., Boca Raton, FL |
| 9. |
Mabey, D.,
and Bailey, R.
(1999)
Br. J. Ophthalmol.
83,
1261-1263 |
| 10. |
Belland, R. J.,
Scidmore, M. A.,
Crane, D. D.,
Hogan, D. M.,
Whitmire, W.,
McClarty, G.,
and Caldwell, H. D.
(2001)
Proc. Natl. Acad. Sci. U. S. A.
98,
13984-13989 |
| 11. | Brunham, R. C. (1999) in Chlamydia Intracellular Biology, Pathogenesis, and Immunity (Stephens, R. S., ed) , pp. 211-238, American Society for Microbiology, Washington, D. C. |
| 12. | Kim, S. K., and DeMars, R. (2001) Curr. Opin. Immunol. 13, 429-436[CrossRef][Medline] [Order article via Infotrieve] |
| 13. | Loomis, W. P., and Starnbach, M. N. (2002) Curr. Opin. Microbiol. 5, 87-91[CrossRef][Medline] [Order article via Infotrieve] |
| 14. | Beatty, W. L., Morrison, R. P., and Byrne, G. I. (1994) Microbiol. Rev. 58, 686-699[Abstract] |
| 15. | Beatty, W. L., Belanger, T. A., Desai, A. A., Morrison, R. P., and Byrne, G. I. (1994) Infect. Immun. 62, 3705-3711[Abstract] |
| 16. |
Taylor, M. W.,
and Feng, G. S.
(1991)
FASEB J.
5,
2516-2522 |
| 17. | Boehm, U., Klamp, T., Groot, M., and Howard, J. C. (1997) Annu. Rev. Immunol. 15, 749-795[CrossRef][Medline] [Order article via Infotrieve] |
| 18. |
Stephens, R. S.,
Kalman, S.,
Lammel, C.,
Fan, J.,
Marathe, R.,
Aravind, L.,
Mitchell, W.,
Olinger, L.,
Tatusov, R. L.,
Zhao, Q.,
Koonin, E. V.,
and Davis, R. W.
(1998)
Science
282,
754-759 |
| 19. |
Read, T. D.,
Brunham, R. C.,
Shen, C.,
Gill, S. R.,
Heidelberg, J. F.,
White, O.,
Hickey, E. K.,
Peterson, J.,
Utterback, T.,
Berry, K.,
Bass, S.,
Linher, K.,
Weidman, J.,
Khouri, H.,
Craven, B.,
Bowman, C.,
Dodson, R.,
Gwinn, M.,
Nelson, W.,
DeBoy, R.,
Kolonay, J.,
McClarty, G.,
Salzberg, S. L.,
Eisen, J.,
and Fraser, C. M.
(2000)
Nucleic Acids Res.
28,
1397-1406 |
| 20. | Kalman, S., Mitchell, W., Marathe, R., Lammel, C., Fan, J., Hyman, R. W., Olinger, L., Grimwood, J., Davis, R. W., and Stephens, R. S. (1999) Nat. Genet. 21, 385-389[CrossRef][Medline] [Order article via Infotrieve] |
| 21. |
Shirai, M.,
Hirakawa, H.,
Kimoto, M.,
Tabuchi, M.,
Kishi, F.,
Ouchi, K.,
Shiba, T.,
Ishii, K.,
Hattori, M.,
Kuhara, S.,
and Nakazawa, T.
(2000)
Nucleic Acids Res.
28,
2311-2314 |
| 22. | Shaw, A. C., Christiansen, G., Roepstorff, P., and Birkelund, S. (2000) Microbes Infect 2, 581-592[CrossRef][Medline] [Order article via Infotrieve] |
| 23. | Miles, E. W. (1991) Adv. Enzymol. Relat. Areas Mol. Biol. 64, 93-172[Medline] [Order article via Infotrieve] |
| 24. | Miles, E. W. (1995) Subcell. Biochem. 24, 207-254[Medline] [Order article via Infotrieve] |
| 25. | Miles, E. W. (2001) Chem. Rec. 1, 140-151[CrossRef][Medline] [Order article via Infotrieve] |
| 26. | Lane, A. N., and Kirschner, K. (1983) Eur. J. Biochem. 129, 571-582[Abstract] |
| 27. |
Miles, E. W.,
and McPhie, P.
(1974)
J. Biol. Chem.
249,
2852-2857 |
| 28. | Crawford, I. P., and Yanofsky, C. (1958) Proc. Natl. Acad. Sci. U. S. A. 44, 1161-1170 |
| 29. | Tipples, G., and McClarty, G. (1991) J. Bacteriol. 173, 4932-4940[Medline] [Order article via Infotrieve] |
| 30. | Caldwell, H. D., Kromhout, J., and Schachter, J. (1981) Infect. Immun. 31, 1161-1176[Medline] [Order article via Infotrieve] |
| 31. | Lacy, M. J., and Voss, E. W., Jr. (1986) J. Immunol. Methods 87, 169-177[CrossRef][Medline] [Order article via Infotrieve] |
| 32. | Smith, O. H., and Yanofsky, C. (1962) Methods Enzymol. 5, 794-806 |
| 33. |
Miles, E. W.
(1970)
J. Biol. Chem.
245,
6016-6025 |
| 34. | Crawford, I. P., Nichols, B. P., and Yanofsky, C. (1980) J. Mol. Biol. 142, 489-502[Medline] [Order article via Infotrieve] |
| 35. |
Fluri, R.,
Jackson, L. E.,
Lee, W. E.,
and Crawford, I. P.
(1971)
J. Biol. Chem.
246,
6620-6624 |
| 36. | Tanizawa, K., and Miles, E. W. (1983) Biochemistry 22, 3594-3603[Medline] [Order article via Infotrieve] |
| 37. |
Miles, E. W.,
Kawasaki, H.,
Ahmed, S. A.,
Morita, H.,
and Nagata, S.
(1989)
J. Biol. Chem.
264,
6280-6287 |
| 38. |
Higgins, W.,
Miles, E. W.,
and Fairwell, T.
(1980)
J. Biol. Chem.
255,
512-517 |
| 39. |
Hyde, C. C.,
Ahmed, S. A.,
Padlan, E. A.,
Miles, E. W.,
and Davies, D. R.
(1988)
J. Biol. Chem.
263,
17857-17871 |
| 40. | Nichols, B. P., and Yanofsky, C. (1979) Proc. Natl. Acad. Sci. U. S. A. 76, 5244-5248[Abstract] |
| 41. |
Nagata, S.,
Hyde, C. C.,
and Miles, E. W.
(1989)
J. Biol. Chem.
264,
6288-6296 |
| 42. |
Milton, D. L.,
Napier, M. L.,
Myers, R. M.,
and Hardman, J. K.
(1986)
J. Biol. Chem.
261,
16604-16615 |
| 43. |
Shirvanee, L.,
Horn, V.,
and Yanofsky, C.
(1990)
J. Biol. Chem.
265,
6624-6625 |
| 44. |
Allen, M. K.,
and Yanofsky, C.
(1963)
Genetics
48,
1065-1083 |
| 45. | Yanofsky, C., Helinski, D. R., and Maling, B. D. (1961) Cold Spring Harbor Symp. Quant. Biol. 26, 11-24[Medline] [Order article via Infotrieve] |
| 46. |
Yanofsky, C.,
and Horn, V.
(1972)
J. Biol. Chem.
247,
4494-4498 |
| 47. | Weyand, M., and Schlichting, I. (1999) Biochemistry 38, 16469-16480[CrossRef][Medline] [Order article via Infotrieve] |
| 48. | Jones, M. L., Gaston, J. S., and Pearce, J. H. (2001) Microb. Pathog. 30, 299-309[CrossRef][Medline] [Order article via Infotrieve] |
| 49. | Coles, A. M., Reynolds, D. J., Harper, A., Devitt, A., and Pearce, J. H. (1993) FEMS Microbiol. Lett. 106, 193-200[CrossRef][Medline] [Order article via Infotrieve] |
| 50. |
Morrison, R. P.
(2000)
Infect. Immun.
68,
6038-6040 |
| 51. | Stephens, R. S. (1999) in Chlamydia Intracellular Biology, Pathogenesis, and Immunity. (Stephens, R. S., ed) , pp. 9-28, American Society for Microbiology Press, Washington, D. C. |
| 52. | Byrne, G. I., Lehmann, L. K., and Landry, G. J. (1986) Infect. Immun. 53, 347-351[Medline] [Order article via Infotrieve] |
| 53. | Crawford, I. P. (1975) Bacteriol. Rev. 39, 87-120[Medline] [Order article via Infotrieve] |
| 54. | Yanofsky, C. (1960) Bacteriol. Rev. 24, 221-245[Medline] [Order article via Infotrieve] |
| 55. | Rose, J. K., Squires, C. L., Yanofsky, C., Yang, H. L., and Zubay, G. (1973) Nat. New Biol. 245, 133-137[Medline] [Order article via Infotrieve] |
| 56. |
Lim, W. K.,
Sarkar, S. K.,
and Hardman, J. K.
(1991)
J. Biol. Chem.
266,
20205-20212 |
| 57. | Ruvinov, S. B., and Miles, E. W. (1992) FEBS Lett. 299, 197-200[CrossRef][Medline] [Order article via Infotrieve] |
| 58. |
Brzovic, P. S.,
Sawa, Y.,
Hyde, C. C.,
Miles, E. W.,
and Dunn, M. F.
(1992)
J. Biol. Chem.
267,
13028-13038 |
| 59. | Brzovic, P. S., Hyde, C. C., Miles, E. W., and Dunn, M. F. (1993) Biochemistry 32, 10404-10413[Medline] [Order article via Infotrieve] |
| 60. |
Miles, E. W.
(1991)
J. Biol. Chem.
266,
10715-10718 |
| 61. | Schneider, T. R., Gerhardt, E., Lee, M., Liang, P. H., Anderson, K. S., and Schlichting, I. (1998) Biochemistry 37, 5394-5406[CrossRef][Medline] [Order article via Infotrieve] |
| 62. |
Yanofsky, C.
(2000)
J. Bacteriol.
182,
1-8 |
| 63. |
Kamath, A. V.,
and Yanofsky, C.
(1992)
J. Biol. Chem.
267,
19978-19985 |
| 64. | Gibbs, R. S. (1987) Am. J. Obstet. Gynecol. 156, 491-495[Medline] [Order article via Infotrieve] |
| 65. | Larsen, B., and Monif, G. R. (2001) Clin. Infect. Dis. 32, 69-77 |
| 66. | Balows, A. (1991) in The Prokaryotes (Balows, A. , Truper, H. G. , Dworkin, M. , Harder, W. , and Schleifer, K.-H., eds) , pp. 1-4, Springer-Verlag New York Inc., New York |
| 67. | Kunst, F., Ogasawara, N., Moszer, I., Albertini, A. M., Alloni, G., Azevedo, V., Bertero, M. G., Bessieres, P., Bolotin, A., Borchert, S., Borriss, R., Boursier, L., Brans, A., Braun, M., Brignell, S. C., Bron, S., Brouillet, S., Bruschi, C. V., Caldwell, B., Capuano, V., Carter, N. M., Choi, S. K., Codani, J. J., Connerton, I. F., Danchin, A., et al.. (1997) Nature 390, 249-256[CrossRef][Medline] [Order article via Infotrieve] |
| 68. | Bult, C. J., White, O., Olsen, G. J., Zhou, L., Fleischmann, R. D., Sutton, G. G., Blake, J. A., FitzGerald, L. M., Clayton, R. A., Gocayne, J. D., Kerlavage, A. R., Dougherty, B. A., Tomb, J. F., Adams, M. D., Reich, C. I., Overbeek, R., Kirkness, E. F., Weinstock, K. G., Merrick, J. M., Glodek, A., Scott, J. L., Geoghagen, N. S., and Venter, J. C. (1996) Science 273, 1058-1073[Abstract] |
This article has been cited by other articles:
 |
J. Griffin, C. Roshick, E. Iliffe-Lee, and G. McClarty Catalytic Mechanism of Chlamydia trachomatis Flavin-dependent Thymidylate Synthase J. Biol. Chem., February 18, 2005; 280(7): 5456 - 5467. [Abstract] [Full Text] [PDF] |
||||
 |
J. H. Carlson, S. Hughes, D. Hogan, G. Cieplak, D. E. Sturdevant, G. McClarty, H. D. Caldwell, and R. J. Belland Polymorphisms in the Chlamydia trachomatis Cytotoxin Locus Associated with Ocular and Genital Isolates Infect. Immun., December 1, 2004; 72(12): 7063 - 7072. [Abstract] [Full Text] [PDF] |
||||
 |
A. W. Solomon, R. W. Peeling, A. Foster, and D. C. W. Mabey Diagnosis and Assessment of Trachoma Clin. Microbiol. Rev., October 1, 2004; 17(4): 982 - 1011. [Abstract] [Full Text] [PDF] |
||||
 |
J. P. Gomes, W. J. Bruno, M. J. Borrego, and D. Dean Recombination in the Genome of Chlamydia trachomatis Involving the Polymorphic Membrane Protein C Gene Relative to ompA and Evidence for Horizontal Gene Transfer J. Bacteriol., July 1, 2004; 186(13): 4295 - 4306. [Abstract] [Full Text] [PDF] |
||||
 |
R. J. Hogan, S. A. Mathews, S. Mukhopadhyay, J. T. Summersgill, and P. Timms Chlamydial Persistence: beyond the Biphasic Paradigm Infect. Immun., April 1, 2004; 72(4): 1843 - 1855. [Full Text] [PDF] |
||||
 |
K. A. Rzomp, L. D. Scholtes, B. J. Briggs, G. R. Whittaker, and M. A. Scidmore Rab GTPases Are Recruited to Chlamydial Inclusions in Both a Species-Dependent and Species-Independent Manner Infect. Immun., October 1, 2003; 71(10): 5855 - 5870. [Abstract] [Full Text] [PDF] |
||||
 |
G. Xie, N. O. Keyhani, C. A. Bonner, and R. A. Jensen Ancient Origin of the Tryptophan Operon and the Dynamics of Evolutionary Change Microbiol. Mol. Biol. Rev., September 1, 2003; 67(3): 303 - 342. [Abstract] [Full Text] [PDF] |
||||
 |
R. P. Morrison New insights into a persistent problem -- chlamydial infections J. Clin. Invest., June 1, 2003; 111(11): 1647 - 1649. [Abstract] [Full Text] [PDF] |
||||
 |
H. D. Caldwell, H. Wood, D. Crane, R. Bailey, R. B. Jones, D. Mabey, I. Maclean, Z. Mohammed, R. Peeling, C. Roshick, J. Schachter, A. W. Solomon, W. E. Stamm, R. J. Suchland, L. Taylor, S. K. West, T. C. Quinn, R. J. Belland, and G. McClarty Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates J. Clin. Invest., June 1, 2003; 111(11): 1757 - 1769. [Abstract] [Full Text] [PDF] |
||||
 |
I. C. Koo and R. S. Stephens A Developmentally Regulated Two-component Signal Transduction System in Chlamydia J. Biol. Chem., May 9, 2003; 278(19): 17314 - 17319. [Abstract] [Full Text] [PDF] |
||||
 |
R. Seshadri, I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg Complete genome sequence of the Q-fever pathogen Coxiellaburnetii PNAS, April 29, 2003; 100(9): 5455 - 5460. [Abstract] [Full Text] [PDF] |
||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
| All ASBMB Journals | Molecular and Cellular Proteomics |
| Journal of Lipid Research | Biochemistry and Molecular Biology Education |