SPARK II Conference Summary
Hyatt Regency
Hotel
Bethesda, MD
January 20, 2003
Contents
The January 20, 2003, SPARK II Conference was part of a
process that was initiated by the NHLBI in October 2002, when
a select group of accomplished scientists -- the SPARK II
Working Group (see attached membership list) -- was assembled
to assist in identifying important opportunities that the
Institute should address over the next three to five years.
The Conference was arranged to obtain the views of
representatives invited by the three major professional
societies associated with the mission of the NHLBI, i.e., the
American Heart Association, the American Thoracic Society, and
the American Society of Hematology (see attached lists of
attendees).
The objective in bringing representatives of those groups
together was not to elicit their ideas on research directions
that address the separate interests of their respective
societies, but rather to ask them to focus on broad research
themes that transcend the traditional organ-specific domains
of the Institute. In addition to their suggestions for
research themes, the participants were also asked to specify
those enabling approaches that would be needed to pursue those
themes effectively.
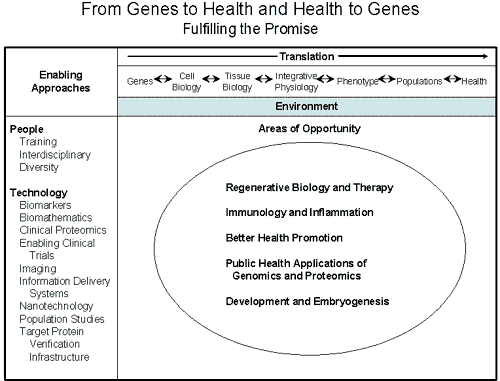
The conference was organized around a modification of the
research schema developed during the SPARK I process. The
modified schema, titled, “From Genes to Health and Health to
Genes – Fulfilling the Promise” (attached), was developed by
the SPARK II Working Group at their earlier meeting. The
research schema included five areas of opportunity and a
number of enabling approaches. The five areas are: 1)
Regenerative Biology and Replacement Therapy, 2) Development
and Embryogenesis, 3) Immunology and Inflammation, 4) Health
Promotion, and 5) Public Health Applications of Genomics and
Proteomics. SPARK II Conference participants were divided up
among four working groups, with Dr. Charles Murry chairing one
addressing the areas of regenerative biology and replacement
therapy and of development and embryogenesis, Dr. James Crapo
chairing one addressing the area of immunology and
inflammation, Dr. Gregory Burke chairing one addressing the
area of health promotion, and Dr. Barry Coller chairing one
addressing the area of public health applications of genomics
and proteomics.
Dr. Valentin Fuster chaired the overall session at which
each of the groups provided a list of suggested research
themes and a summary session that sought to integrate the
results of the separate sessions. The recommendations in the
four areas are presented below. Although some overlap exists
across the recommendations developed in each of the areas,
together they outline important opportunities for the
Institute. As for the development of research capacity, a
consensus emerged in terms of what is needed to ensure
progress in the identified opportunities.
Return
to Contents
Regenerative Biology and Replacement
Therapy/Development and Embryogenesis
Current options for treating damage to heart, lung,
vascular, or blood systems are limited. Stem cells that can be
directed to differentiate into specific cell types offer the
possibility of a renewable source of replacement tissues.
However, careful attention needs to be given to identifying
those conditions that are suitable for regenerative and
replacement therapies and the methodologies that may be used
to implement them. Congestive heart failure, myocardial
infarction, bronchopulmonary dysplasia, cystic fibrosis,
bleeding disorders, and hemoglobinopathies are the most likely
initial candidates, but other conditions, including asthma,
autoimmunity, atherosclerosis, and congenital heart disease,
should also be considered.
Realizing the full therapeutic potential of stem cells will
depend on a better understanding of basic developmental
systems, embryogenesis, in utero environmental biology, and
the epigenetic and other factors that control or reprogram a
cell’s fate. To conduct research in these areas, investigators
will require new research tools, e.g., genomic and proteomic
arrays, agents for inducing and monitoring gene expression,
assays for epigenetics, imaging techniques, and new
quantitative approaches to genetic and genomic networks.
The processes that influence injury and repair in vivo are
likely to vary depending on the organ system(s) involved.
Studies are needed that are focused on the development of
heart muscle, lung, and blood cells from precursors and on
ways to stimulate tissues that do not normally regenerate to
do so. Of particular interest are studies of angiogenesis and
arterial remodeling, processes which will likely be required
for regeneration of any solid tissue.
Combining gene therapy with stem cells may provide an
important new treatment approach for heart, lung, and blood
diseases. Although an increasing number of gene therapy trials
are underway, to advance the field toward general clinical
use, further research is needed on such critical issues as
cell-specific targeting strategies and regulation of gene
expression. Also needed are efforts to develop more effective
vectors and non-vector based approaches to gene replacement
and repair.
Rushing stem cell research prematurely to the clinic could
jeopardize support for future developments in this area. A
measured approach is needed that entails the development and
use of intermediate, clinically relevant animal models for
pharmacology and toxicology studies prior to studies in
humans. Such models would allow researchers to compare
therapies using stem cells from various sources (e.g., bone
marrow, embryonic stem cells, fetal tissue, cord blood). They
can also provide insight on the timing of cell-based
intervention relative to disease stage; the advantages and
disadvantages of transplanting functional, differentiated
cells (e.g., islets in the case of diabetes); the influence of
aging (both donor age and cellular senescence) on transplant
and therapeutic success; and the effectiveness of therapies
based on various cells and combinations of cells.
A parallel effort is needed to develop systems to deliver
cell-based regenerative and replacement therapies to humans
and thereby enable translation of research results from the
laboratory to the clinic. Such approaches are likely to differ
from those used for traditional pharmaceutical therapies.
Centers including both basic and clinical expertise that
conduct clinically relevant research on all aspects of
cell-based therapies should be developed and should be
complemented by an infrastructure of central resources such as
cell processing cores, animal facilities, genomic and
proteomic laboratories, and facilities capable of producing
reagents and purifying cells using good manufacturing
practices. A regulatory coordination center to assist
scientists in navigating FDA requirements would also be a
useful addition.
The Institute should consider efforts to encourage
establishment of a trans-NIH Stem Cell Advisory Committee
(SAC), similar to the Recombinant DNA Advisory Committee
(RAC). The SAC would ensure rigor and accountability in all
human studies of stem-cell based therapies.
Return
to Contents
Immunology and Inflammation
Recent advances suggest that the immune system contributes
to the wide range of manifestations and complexity of
pathogenesis of diseases of the heart, lung, and blood
vessels. Common pathways that lead to disordered inflammatory
and immune responses may contribute to their etiology or
progression. An understanding of the regulation of
inflammatory events as they relate to tissue injury and repair
should provide new insights into potential preventive and
therapeutic interventions for heart, lung, and blood diseases.
One reason that the immune system is so difficult to study
is that many events occur simultaneously. Multiple pathways
create multiple interactions, with numerous regulatory factors
influencing up- or down-regulation. Significant gaps remain in
our understanding of immunity and inflammation including: the
factors that control the shift from a normal state to one of
autoimmunity or allergy; the genetic or environmental
influences that predispose us to develop immune-related
disease; the indicators that predict prognosis and therapeutic
response; and the factors that keep us healthy. Contributing
to our lack of understanding is the scarcity of interaction
between classical immunologists and clinicians. Classical
immunologists define linear pathways marked by limited
interactions between a small, defined set of effectors. They
should be encouraged to take a broader approach because
translation to clinical applications will require
consideration of interactions between multiple pathways under
diverse environmental conditions.
Several unique aspects of the immune and inflammatory
systems are relevant to the mission of the NHLBI. They include
precursor/stem cells, the clotting system, thrombosis,
atherosclerosis, signaling and regulatory elements,
microvascular involvement, and antigen processing in the lung,
where the immune system milieu includes surfactants,
antioxidants, alveolar macrophages, and marginated
polymorphonuclear neutrophils. Conditions directly related to
immunity and inflammation include acute lung injury, asthma,
autoimmune disease, atherosclerosis and plaque rupture,
cardiomyopathy, chronic obstructive pulmonary disease,
infections, interstitial lung disease, microvascular disease,
sepsis, thrombosis, transplant rejection, transfusion
complications, trauma, and valvular heart disease.
One way to address diseases related to immunology and
inflammation would be to create a program of immunomodulatory
centers. Unlike existing centers programs, the centers in the
proposed program would not be constrained to single
institutions or even to limited geographical areas, but
instead would be encouraged to include investigators
throughout the country at multiple institutions and would
serve as national cores focused on multiple diseases. They
would encourage collaboration through network interactions,
provide investigators greater access to large sample groups,
expand training to a national base, and create better
standardization in data and sample collection. As an
alternative to developing a new mechanism of support, the
Institute could achieve the objectives envisioned for the
proposed centers program by using the program project grant
(PPG) mechanism to solicit programs that are nationally based
rather than institution based and that would encourage the
inclusion of both clinical and training components.
Further technological developments are needed in the areas
of large-scale assays, biomarkers, molecular imaging,
nanotechnology, genomics, proteomics, computational biology,
and epidemiology. This will require involvement of individuals
skilled in a range of disciplines not usually associated with
research on immunology and inflammation, including
bioengineering. New animal models may be necessary as an
intermediate step between research in mice or other small
animal models and humans. Also needed are new methods for
noninvasive data collection and integrative models that
reflect human conditions. Partnerships with private industry
should be developed relating to toxicology testing and good
manufacturing practice for synthesis of potential
therapeutics.
Return
to Contents
Health Promotion
Health promotion can be defined broadly as helping
individuals and populations achieve optimal levels of
wellness, with particular emphasis on reaching those who are
most vulnerable and fostering good health across the life
span. Within this context, goals of the NHLBI are (1) to
achieve better health outcomes, at both the population level
and the clinical practice level, and (2) to reduce the gap
between what we know and what we deliver in both public health
programs and clinical care, while continuing to generate new
knowledge.
Although the scientific basis for many health-promotion
recommendations is well recognized and undisputed, this
knowledge has not translated into the hoped-for public health
improvements. Future progress toward achieving health
promotion objectives will hinge on an integrated approach that
involves not only the expertise of bench scientists,
epidemiologists, and behavioral scientists, but also strong
collaboration with health care systems managers, educational
experts, sociologists, marketers, policymakers/legislators,
and representatives of government, industry, and lay groups.
The forces that conspire to undermine health in this country
are not just personal but societal, and a broad-based effort
is needed to overcome them.
Behavior change is essential if we are to reduce the
prevalence of lifestyle risk factors and to achieve compliance
with medical regimens. Unfortunately, research has yet to
identify means of modifying behavior that can be easily and
widely implemented. Studies are needed to develop and test new
strategies and, conversely, to pinpoint the reasons for
failures of current approaches. Emotions and personality
characteristics strongly influence behavior, and
multifactorial interventions that address physical risk
factors (e.g., smoking) within the context of psychosocial
risk factors (e.g., depression) constitute promising avenues
for exploration. Similarly, understanding why certain risk
factors (e.g., obesity, diabetes, hypertension) tend to
cluster may shed important light on vulnerability to risk and
how it can be addressed. Basic research to elucidate genetic
predispositions to behaviors may ultimately provide a basis
for individualized interventions to prevent risk factors from
developing in the first place (so-called primordial
prevention).
In addition to individual characteristics, policy and
environmental factors strongly influence health status. The
health impact of policies outside the health arena (e.g.,
transportation, smoking, taxes) needs to be better
appreciated. Interventions such as changes in food advertising
or packaging, neighborhood characteristics, and worksite- or
school-based settings should be evaluated. Researchers should
examine models from the business world (e.g., the successful
marketing of fast foods) for insight into how the tastes and
preferences of the public can be shaped. Similarly, much can
be learned from professional journalists and other media
experts about how to communicate clear and persuasive messages
to the public.
In general, our health-care delivery system is ill equipped
to move ahead with health promotion. Good models of systems
that effectively promote health (as opposed to treat disease)
are lacking. We need to encourage development of clinical
practice research groups and make them accessible to
researchers. Translational research to move from efficacy to
effectiveness should be a high priority, including
community-based demonstration projects in ethnically,
geographically, and economically diverse groups across the
life span. Such studies should determine which strategies are
most effective in promoting adherence to preventive
recommendations in the community, across health-care
setting/organizations, by different types of providers, and by
various patients/consumers. They should also examine the
biases, selection problems, intervention intensities, and
other factors that result in failure of study outcomes to
translate into real-world outcomes. Close attention should be
paid to the roles of nonphysician health care professionals
(e.g., nurses, physician assistants, psychologists). Efforts
must also be made to integrate public health messages so that
they do not conflict with one another and thereby overwhelm
their audiences and lose impact.
Return
to Contents
Public Health Applications of Genomics
and Proteomics
The challenge is to improve medical practice and public
health by developing innovative ways to use the large volumes
of genomic and proteomic information that have already been
assembled and continue to be generated. On the immediate
horizon are clinical advances that should allow doctors to
assess quickly the genome of patients and identify selected
genetic variants and the proteins they express. This should
allow diagnosis, drug selection, and drug dosing to be
tailored to individual patients. It should also increase the
effectiveness of preventative medicine, allowing doctors to
screen patients for genetic diseases, determine their
susceptibility, and then provide interventions before symptoms
are manifest. However, advances in patient care using genomic
and proteomic science are being developed much more slowly
than advances in basic science. Therefore, the Institute
should focus on ways to use existing resources to translate
ideas and preliminary data into practice and to ensure that
both the investigators and the samples they will need for
continued discovery will be available.
Major obstacles to translating genomic and proteomic
medicine include: 1) inadequate access to tissue and
phenotypic data for researchers trying to study variations
within populations, 2) limited support for interdisciplinary
research and pilot studies, and 3) a possible lack of future
physician scientists with appropriate training.
The Institute should encourage investigators to move beyond
the study of serial signaling cascades and single-gene
diseases and focus instead on complex biological systems and
the properties that are associated with them. New technologies
such as gene and protein chips, mass spectrometry, and
bioinformatics should make this possible by allowing
researchers to investigate protein-protein, protein-DNA, and
protein-small molecule interactions more
efficiently.
Small, "proof of concept" projects may
provide a way to support the translation of selected basic
research results into clinically applicable therapies. This
could produce models that could be followed by other
investigators in developing genomic applications as well as
help identify existing impediments to such development.
Development grants (i.e., R21s) with a focus on determining
normal population genetic variations and exploring
applications of new genomic and proteomic technologies would
also be valuable. The potential for research that links
genomics, nutrition, and pharmacology should also be
considered.
The Institute is encouraged to build upon existing programs
as an effective way to capitalize on new opportunities.
Investigators should be provided with improved access to the
samples collected in completed trials and established studies
so that they can be examined for genetic markers in new
disease areas. The Institute should also explore ways to use
its Programs for Genomic Applications and its Proteomics
Initiative to educate scientists about the available resources
and to allay concerns in the general public about potential
misuse of genetic data. Possible approaches to increasing the
value of the existing genomics and proteomics programs include
encouraging or requiring them to provide classes in genomics
and proteomics for NHLBI-funded trainees and fellows and to
design workshops to inform scientists about resources and
technologies available to them through the NHLBI.
In order to encourage the inclusion of one or more genomic
or proteomic components in new clinical trials, investigators
may need incentives such as supplemental funding or access to
collaborative expertise. A Phenotyping and Tissue Collection
Network also merits consideration. It would be a long-term,
prospective program to make available to individual
investigators carefully collected biological samples and data,
thereby enabling individual investigators to examine much
larger collections of samples than they could ever assemble on
their own. In addition, consideration should be given,
although with lower priority and only if resources are
plentiful, to a Genomic or Proteomic Centers program that
would be similar to the Institute's existing programs, but
would have an additional focus on translating genomic and
proteomic discoveries to clinical applications. The centers
would be interdisciplinary and would conduct research on
integrative biology and human applications, as well as provide
community education and outreach.
Finally, the need for technological advances to promote the
translation of genomic and proteomic information into public
health benefits must be recognized. A parallel improvement in
phenotyping is also needed, and will require improved imaging
methods, particularly in molecular imaging, and refinements in
biomarker technology.
Return
to Contents
Enabling Approaches
People
Support should continue to be provided for debt repayment
and consideration should be given to expanding the program to
attract additional physicians to research careers and perhaps
to extending it to cover bench scientists as well.
Innovative approaches are needed to attract investigators
trained in disciplines other than those usually involved in
research supported by the Institute. Research on health
promotion would benefit from participation by experts in areas
such as marketing, psychology, sociology, political science,
and journalism. Cross-cultural training for investigators
focused on health promotion will be particularly important.
Programs that emphasize dual degrees, such as MD/MPH, MD/PhD,
RN/MPH, or PhD/MPH would help to ensure research progress.
Multidisciplinary training and interdisciplinary teams will
be critical in developing the capacity to conduct research in
all of the areas identified by the SPARK II Working Group,
particularly to conduct stem cell or gene therapy clinical
trials. Cells are not the same as molecules, and clinicians
are going to need new skill sets for performing and evaluating
stem cell transplants and gene therapy approaches. Training
for bioengineers needs to be integrated with medical school
curricula – the "bio" in “bioengineering" needs to be
emphasized.
Consideration should be given to revising the current
constraints on salary levels on career development awards. A
career development award program for PhDs should also be
considered.
Central to all of the training and career development
programs of the Institute must be a recognition that
additional time may be required to develop the broad skills
necessary to conduct research in these complex areas. Also
needed will be increased emphasis on the quality and duration
of mentoring. Innovative ways to extend training experiences
should be explored, including the provision of supplements to
training grants for education in translation research.
Attention needs to be directed to developing ways of
assisting young investigators in establishing research
careers. It is a widespread perception that investigators
applying for their first research grants do not fare as well
in the review process as experienced researchers, despite
review guidelines that ask reviewers to hold the new
applicants to less stringent standards for preliminary
data.
Other
Standing review groups tend not to encompass the full range
of expertise necessary to review complex, multidisciplinary
research protocols and proposals to conduct translational
research. Although the Institute cannot address this issue for
investigator-initiated research, it should ensure that review
groups assembled for Institute-solicited research are broadly
representative.
Return
to Contents
From Genes to Health and Health to
Genes
Return
to Contents
SPARK II Working Group
Claude Lenfant, M.D.,
Chair
Director
National Heart, Lung, and Blood
Institute
Bethesda, Maryland 20892
Gregory L. Burke, M.D., M.S.
Professor and Chairman,
Department of Public Health Sciences
Wake Forest
University School of Medicine
Winston-Salem, NC
27157-1063
Barry S. Coller, M.D.
David Rockefeller Professor of
Medicine
The Rockefeller University
New York, NY
10021
James D. Crapo, M.D.
Executive Vice President,
Academic Affairs
National Jewish Medical and Research
Center
Denver, CO 80206
Valentin Fuster, M.D.
Professor of Medicine
Mount
Sinai School of Medicine
New York, NY 10029
Charles E. Murry, M.D., Ph.D.
Professor
Pathology
University of Washington School of
Medicine
Seattle, WA 98195
Judith L. Swain, M.D.
Chair, Department of
Medicine
Stanford University
Stanford, CA 94305
Samuel Wickline, M.D.
Professor of Medicine and
Biomedical Engineering
Co-Director, Cardiovascular
Division
Washington University School of Medicine
St.
Louis, MO 63110
Redford B. Williams, Jr., M.D.
Director, Behavioral
Medicine Research Center
Duke University Medical
Center
Durham, NC 27710
American Heart Association
Dianne L. Atkins, M.D.
University of Iowa
Iowa
City, IA 52242-1083
Roberto Bolli, M.D.
University of
Louisville
Louisville, KY 40202
Robert O. Bonow, M.D.
Northwestern University Medical
School
Chicago, IL 60611
Timothy J. Gardner, M.D.
Hospital of the University of
Pennsylvania
Philadelphia, PA 19104-4206
David C. Goff, Jr., M.D., Ph.D.
Wake Forest University
School of Medicine
Winston-Salem, NC 27157
Augustus Grant, M.D., Ph.D.
Duke University Medical
Center
Durham, NC 27710
Philip Greenland, M.D.
Northwestern University Medical
School
Chicago, IL 60611-4480
Laura L. Hayman, Ph.D.
New York University
New
York, NY 10003-6677
David M. Herrington, M.D.
Wake Forest University
School of Medicine
Winston-Salem, NC 27157-1045
Daniel W. Jones , M.D.
University of Mississippi
Medical Center
Jackson, MS 39216-4505
Ronald M. Krauss, M.D.
Children's Hospital Oakland
Research Institute
Oakland, CA 94609
Eduardo Marban, M.D., Ph.D.
Johns Hopkins
University
Baltimore, MD 21205-2109
Rose Marie Robertson, M.D.
American Heart
Association
Dallas, TX 75231-4596
Sidney Smith, M.D.
University of North Carolina at
Chapel Hill
Chapel Hill, NC 27599
James A. Weyhenmeyer, Ph.D.
University of
Illinois
Urbana, IL 61801
American Thoracic Society
David M. Center, M.D.
Boston University
School of Medicine
Boston, MA 02118
Jeffrey L. Curtis, M.D.
University of Michigan
Ann
Arbor, MI 48105
J. Randall Curtis, M.D.
University of
Washington
Seattle, WA 98104
Claire M. Doerschuk, M.D.
Case Western Reserve
University
Cleveland, OH 44106
Mark W. Frampton, M.D.
University of Rochester Medical
Center
Rochester, NY 14642-8692
Jeffrey J. Fredberg, Ph.D.
Harvard School of Public
Health
Boston, MA 02115-6021
David Gozal, M.D.
University of Louisville School of
Medicine
Louisville, KY 40202-1788
Susan Janson, Ph.D.
University of California at San
Francisco
San Francisco, CA 94143-0608
Alan H. Jobe, M.D., Ph.D.
Children's Hospital Medical
Center
Cincinnati, OH 45229-3026
Andrew H. Limper, M.D.
Mayo Clinic and
Foundation
Rochester, MN 55905
Thomas R. Martin, M.D.
VA Puget Sound Health Care
System
Seattle, WA 98108
Sadis Matalon, Ph.D.
University of Alabama at
Birmingham
Birmingham, AL 35233-6810
Bruce R. Pitt, Ph.D.
University of Pittsburgh Graduate
School of Public Health
Pittsburgh, PA 15260
Dean Sheppard, M.D.
University of California at San
Francisco
San Francisco, CA 94143-0854
Peter D. Wagner, M.D.
University of California at San
Diego Medical Center
La Jolla, CA 92093-5004
American Society of Hematology
Kenneth A. Bauer, M.D.
VA Medical Center
West
Roxbury, MA 01232
James B. Bussel, M.D.
Weill Cornell Medical
Center
New York, NY 10021
George Daley, M.D., Ph.D.
Whitehead
Institute
Cambridge, MA 02142
Charles Esmon, Ph.D.
Howard Hughes Medical
Institute
Oklahoma City, OK 73104
Barbara C. Furie, Ph.D.
Beth Israel Deaconess Medical
Center
Boston, MA 02215
Todd R. Golub, M.D.
Dana-Farber Cancer
Institute
Boston, MA 02115
Robert I. Handin, M.D.
Brigham and Women's
Hospital
Boston, MA 02115
Robert P. Hebbel, M.D.
University of
Minnesota
Minneapolis, MN 55455
Katherine A. High, M.D.
The Children's Hospital of
Philadelphia
Philadelphia, PA 19104-4318
Ronald Hoffman, M.D.
University of
Illinois-Chicago
Chicago, IL 60607-4004
Jay E. Menitove, M.D.
Community Blood Center
Kansas
City, MO 64111
Stuart H. Orkin, M.D.
Dana Farber Cancer
Institute
Boston, MA 02115
Elliott Vichinsky, M.D.
Children's Hospital Medical
Center
Oakland, CA 94609
Return
to Contents
|
